Abstract
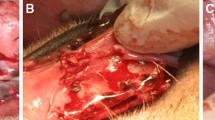
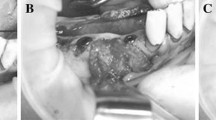
This work assessed the performance of membranes made of natural latex extracted from Hevea brasiliensis prepared with three different methods: polymerized immediately after collection without the use of ammonia (L1); polymerized after preservation in ammonia solution (L2); and polymerized after storage in ammonia, followed by Soxhlet technique for the extraction of substances (L3). Polytetrafluoroethylene (PTFE) membrane was used as control. Two 10-mm diameter bone defects were surgically made in the calvaria of thirty adult male New Zealand rabbits. Defects (total n = 60) were treated with guided bone regeneration (GBR) using L1, L2, L3 or PTFE membranes (n = 15 for each membrane). Ten animals were euthanized after 7, 20 and 60 days postoperatively so that five samples (n = 5) of each treatment were collected at each time, and bone regeneration was assessed microscopically. The microscopic analysis revealed defects filled with blood clot and new bone formation at the margins of the defect in all 7-day samples, while 20-day defects were mainly filled with fibrous connective tissue. After 60 days defects covered with L1 membranes showed a significantly larger bone formation area in comparison to the other groups (P < 0.05, ANOVA, Tukey). Additionally, bone tissue hypersensitization for L1 and PTFE membranes was also investigated in six additional rabbits. The animals were subjected to the same surgical procedure for the confection of one 10-mm diameter bone defect that was treated with L1 (n = 3) or PTFE (n = 3). Fifty-three days later, a second surgery was performed to make a second defect, which was treated with the same type of membrane used in the first surgery. Seven days later, the animals were euthanized and samples analyzed. No differences among L1 and PTFE samples collected from sensitized and non-sensitized animals were found (P > 0.05, Kruskal–Wallis). Therefore, the results demonstrated that latex membranes presented performance comparable to PTFE membranes, and that L1 membranes induced higher bone formation. L1 and PTFE membranes produced no hypersensitization in the bone tissue.






Similar content being viewed by others
References
Buser D. 20 years of guided bone regeneration in implant dentistry. 2nd ed. Chicago: Quintessence; 2010.
Dimitriou R, Jones E, McGonagle D, Giannoudis P. Bone regeneration: current concepts and future directions. BMC Med. 2011;9(1):66.
Ferguson C, Alpern E, Miclau T, Helms JA. Does fracture repair recapitulate skeletal formation? Mech Dev. 1999;87(1–2):57–66.
Shapiro F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur. Cells Mater. 2008;15:53–76.
Nyman S, Lindhe J, Karring T, Rylander H. New attachment following surgical treatment of human periodontal disease. J Clin Periodontol. 1982;9(4):290–6.
Dahlin C, Linde A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg. 1988;81(5):672–6.
Nyman S. Bone regeneration using the principle of guided tissue regeneration. 1991;18(6):494–8.
Linde A, Alberius P, Dahlin C, Bjurstam K, Sundin Y. Osteopromotion: a soft-tissue exclusion principle using a membrane for bone healing and bone neogenesis. J Periodontol. 1993;64(11 Suppl):1116–28.
Buser D, Hirt HP, Schenk RK. Lateral ridge augmentation using autografts and barrier membranes: a clinical study with 40 partially edentulous patients. J Oral Maxillofax Surg. 1996;54(4):420–32.
Monteiro ASF, et al. Polyurethane and PTFE membranes for guided bone regeneration: histopatological and ustrastructural evaluation. Med Oral Patol Oral Cir Bucal. 2010;15(2):e401–6.
Retzepi M, Donos N. Guided bone regeneration: biological principles and therapeutic applications. Clin Oral Impl Res. 2010;21(6):567–76.
Balabanian CACA, Coutinho-Netto J, Lamano-Carvalho TL, Lacerda SA, Brentegani LG. Biocompatibility of natural latex implanted into dental alveolys of rats. J Oral Sci. 2006;48(4):201–5.
Ereno C, Guimarães SAC, Pasetto S, Herculano RD, Silva CP, Graeff CFO, Tavanno O, Baffa O, Kinoshita A. Latex use as an occlusive membrane for guided bone regeneration. J Biomed Mat Res. 2010;95(3):932–9.
Floriano J, Mota L, Furtado E, Rossetto V, Graeff CO. Biocompatibility studies of natural rubber latex from different tree clones and collection methods. J Mater Sci Mater Med. 2013;16(1):1–10.
Andrade TA, et al. The inflammatory stimulus of a natural latex biomembrane improves healing in mice. Braz J Med Biol Res. 2011;44(10):1036–47.
Mendonça RJ, Maurício VB, de Bortolli Teixeira L, Lachat JJ, Coutinho-Netto J. Increased vascular permeability, angiogenesis and wound healing induced by the serum of natural latex of the rubber tree Hevea brasiliensis. Phytother Res. 2010;24(5):764–8.
Mengumpun K, et al. Hidrophobic allergens from the bottom fraction membrane of Hevea brasiliensis. Asian Pac J Allergy Immunol. 2008;26(2–3):129–36.
Gawchik SM. Latex Allergy. Mt Sinai J Med. 2011;78(5):759–72.
Deval R, Ramesh V, Prasad G, Jain AK. Natural rubber latex allergy. Indian J Dermatol Venereol Leprol. 2008;74(4):304–10.
Janet CG, Barbee RW, Bielitzki JT, Clayton LA, Donovan JC, Hendriksen CFM, Kohn DF, Lipman NS, Locke PA, Melcher J, Quimby FW, Turner PV, Wood GA, Würbel H. Guide for the care and use of laboratory animals. 8th ed. Washington DC: National Academies Press; 2011.
Yaltirik M, Ozbas H, Bilgic B, Issever H. Reactions of connective tissue to mineral trioxide aggregate and amalgam. J Endod. 2004;30(2):95–9.
Internationale FD. Recommended Standard practices for biological evaluation of dental materials, Part 4. 11: subcutaneous implantation test. Int Dent J. 1980;30:173–4.
Marques L, Holgado LA, Simoes RD, Pereira JD, Floriano JF, Mota LS, Graeff CFO, Constantino CJL, Rodriguez-Perez MA, Matsumoto M, Kinoshita A. Subcutaneous tissue reaction and cytotoxicity of polyvinylidene fluoride and polyvinylidene-trifluoroethylene blends associated with natural polymers. J Biomed Mater Res B Appl Biomater. 2013;101(7):1284–93.
Gomes-Filho JE, de Moraes Costa MT, Cintra LTÂ, Lodi CS, Duarte PCT, Okamoto R, et al. Evaluation of alveolar socket response to Angelus MTA and experimental light-cure MTA. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2010;110(5):e93–7.
Dahlin C, et al. Healing of maxillary and mandibular bone defects using a membrane technique. An experimental study in monkeys. Scand Plast Reconstr Surg Hand Surg. 1990;24(1):13–9.
Brandão ML, CoutinhoNetto J, Thomazinin JA, Lachat JJ, Muglia VF, Piccinato CE. Prótese vascular derivada do látex. J Vasc Bras. 2007;6(2):130–41.
Talieri IC, et al. Enxerto de látex natural na cicatrização de esclerectomias lamelar e penetrante em coelhos. Ciência Rural Santa Maria. 2009;39(6):1815–22.
Zimmerman M. Teste de biocompatibilidade e resistência de membranas de látex em cães. Ciência Rural Santa Maria. 2007;37(6):1719–23.
Herculano RD, Silva CP, Ereno C, Guimaraes SAC, Kinoshita A, Graeff CFO. Natural rubber latex used as drug delivery system in guided bone regeneration (GBR). Mater Res. 2009;12:253–6.
Herculano RD, Guimarães SAC, Belmonte GC, Duarte MAH, Oliveira Júnior ON, Kinoshita A, Graeff CFO. Metronidazole release using natural rubber latex as matrix. Mater Res. 2010;13:57–61.
Herculano RD, Alencar de Queiroz AA, Oliveira ON Jr, Graeff CFO. On the release of metronidazole from natural rubber latex membranes. Mater Sci Eng. 2011;31(2):272–5.
Frade MAC, Cursi IB, Andrade FF, Coutinho Netto J, Barbetta FM, Foss NT. Manegement of diabetic skin wounds with a natural latex biomembrane. Med Cutan Iber Lat Am. 2004;32(4):157–62.
Sampaio RB, Mendonca RJ, Simioni AR, Costa RA, Siqueira RC, Correa VM, Tedesco AC, Haddad A, Coutinho Netto J, Jorge R. Rabbit retinal neovascularization induced by latex angiogenic-derived fraction: an experimental model. Curr Eye Res. 2010;35(1):56–62.
Lindhe J, Karring T, Lang NP. Clinical periodontology and implant dentistry. 5th ed. Oxford: Wiley-Blackwell; 2008.
Ferreira M, Mendonça RJ, Coutinho-Netto J, Mulato M. Angiogenic properties of natural rubber latex biomembranes and the serum fraction of Hevea brasiliensis. Braz J Phys. 2009;39:564–9.
Kaigler D, Silva E, Mooney DJ. Guided bone regeneration using injectable vascular endothelial growth factor delivery gel. J Periodontol. 2013;84(2):230–8.
Gu Z, Xie H, Li L, Zhang X, Liu F, Yu X. Application of strontium-doped calcium polyphosphate scaffold on angiogenesis for bone tissue engineering. J Mater Sci Mater Med. 2013;24(5):1251–60.
Rippel MM, Leite CAP, Lee L-T, Galembeck F. Formation of calcium crystallites in dry natural rubber particles. J Colloid Interface Sci. 2005;288(2):449–56.
Mrué F, et al. Evaluation of the biocompatibility of a new biomembrane. Mater Res. 2004;7(2):277–83.
Abbas AK, Lichtman AHH, Pillai S. Imunologia celular e molecular. Rio de Janeiro: Elsevier Brasil; 2012.
Gawchik SM. Latex Allergy. Mount Sinai J Med. 2011;78(5):759–72.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moura, J.M.L., Ferreira, J.F., Marques, L. et al. Comparison of the performance of natural latex membranes prepared with different procedures and PTFE membrane in guided bone regeneration (GBR) in rabbits. J Mater Sci: Mater Med 25, 2111–2120 (2014). https://doi.org/10.1007/s10856-014-5241-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-014-5241-1