Abstract
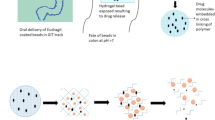
The purposes of this study were to develop and evaluate calcium pectinate/alginate microspheres (PAMs) and to exploit their pH-sensitive properties for colon-targeted delivery of encapsulated cisplatin. PAMs were prepared using an electrospraying method. The PAMs, as cores, were then coated with Eudragit S100 using a polyelectrolyte multilayer coating technique in aqueous solution. The morphology of the microspheres was observed under scanning electron microscopy. In vitro drug release studies were performed in simulated gastrointestinal fluid, and the results indicated that approximately 5 % of the cisplatin was released from the Eudragit S100-coated PAMs, and 51 % of the cisplatin was released from the uncoated PAMs at 1 h. The release of cisplatin from the Eudragit S100-coated PAMs was more sustained in simulated gastric fluid than in simulated intestinal fluid due to the increased solubility of the coating polymer in media with pH >7.0. Drug release from the Eudragit S100-coated PAMs was best described by the Higuchi’s square root model. From these results, it was concluded that Eudragit S100-coated PAMs are a potential carrier for delivery of cisplatin to the colon.





Similar content being viewed by others
References
Zhang L, Cao F, Ding B, Li Q, Xi Y, Zhai G. Eudragit® S100 coated calcium pectinate microspheres of curcumin for colon targeting. J Microencapsul. 2011;28:659–67.
Pruefer FG, Lizarraga F, Maldonado V, Melendez-Zajgla J. Participation of Omi Htra2 serine-protease activity in the apoptosis induced by cisplatin on SW480 colon cancer cells. J Chemother. 2008;20:348–54.
Urien S, Brain E, Bugat R, Pivot X, Lochon I, Van ML, Vauzelle F, Lokiec F. Pharmacokinetics of platinum after oral or intravenous cisplatin: a phase 1 study in 32 adult patients. Cancer Chemother Pharmacol. 2005;55:55–60.
Chourasia MK, Jain SK. Pharmaceutical approaches to colon targeted drug delivery systems. J Pharm Pharm Sci. 2003;6:33–66.
Vaidya A, Jain A, Khare P, Agrawal RK, Jain SK. Metronidazole loaded pectin microspheres for colon targeting. J Pharm Sci. 2009;98:4229–36.
Philip AK, Philip B. Colon targeted drug delivery systems: a review on primary and novel approaches. Oman Med J. 2010;25:79–87.
Tsai SW, Chen CC, Liou HM, Hsu FY. Preparation and characterization of microspheres comprised of collagen, chondroitin sulfate, and apatite as carriers for the osteoblast-like cell MG63. J Biomed Mater Res A. 2010;93:115–22.
Salyers AA, Vercellotti JR, West SE, Wilkins TD. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977;33:319–22.
Shah N, Shah T, Amin A. Polysaccharides: a targeting strategy for colonic drug delivery. Expert Opin Drug Deliv. 2011;8:779–96.
Ribeiro AJ, Silva C, Ferreira D, Veiga F. Chitosan-reinforced alginate microspheres obtained through the emulsification/internal gelation technique. Eur J Pharm Sci. 2005;25:31–40.
Kushwaha P, Fareed S, Nanda S, Mishra A. Design & fabrication of tramadol HCl loaded multiparticulate colon targeted drug delivery system. J Chem Pharm Res. 2011;3:584–95.
Khan MZ, Prebeg Z, Kurjaković N. A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers. I. Manipulation of drug release using Eudragit L100-55 and Eudragit S100 combinations. J Control Release. 1999;58:215–22.
Vaidya A, Jain A, Khare P, Agrawal RK, Jain SK. Metronidazole loaded pectin microspheres for colon targeting. J Pharm Sci. 2009;98:4229–36.
Rahman Z, Kohli K, Khar RK, Ali M, Charoo NA, Shamsher AA. Characterization of 5-fluorouracil microspheres for colonic delivery. AAPS PharmSciTech. 2006;7:E47.
Golla ED, Ayres GH. Spectrophotometric determination of platinum with o-phenylenediamine. Talanta. 1973;20:199–210.
Obeidat WM, Price JC. Preparation and evaluation of Eudragit S100 microspheres as pH-sensitive release preparations for piroxicam and theophylline using the emulsion-solvent evaporation method. J Microencapsul. 2006;23:195–202.
Rawat M, Saraf S, Saraf S. Influence of selected formulation variables on the preparation of enzyme-entrapped Eudragit S100 microspheres. AAPS PharmSciTech. 2007;8:E116.
Perumal D. Microencapsulation of ibuprofen and Eudragit RS 100 by the emulsion solvent diffusion technique. Int J Pharm. 2001;218:1–11.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hsu, FY., Yu, DS. & Huang, CC. Development of pH-sensitive pectinate/alginate microspheres for colon drug delivery. J Mater Sci: Mater Med 24, 317–323 (2013). https://doi.org/10.1007/s10856-012-4798-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-012-4798-9