Abstract
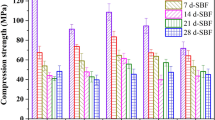
To develop high macroporous and degradable bone cements which can be used as the substitute of bone repairing and drug carriers, cross-linked gelatin microspheres (GMs) and calcium sulfate dihydrate (CSD) powder were incorporated into calcium phosphate bone cement (CPC) to induce macropores, adjust drug release and control setting time of α-TCP–liquid mixtures after degradation of GMs and dissolution of CSD. In this study, CSD was introduced into CPC/10GMs composites to offset the prolonged setting time caused by the incorporation of GMs, and gentamicin sulphate (GS) was chosen as the model drug entrapped within the GMs. The effects of CSD amount on the cement properties, drug release ability and final macroporosity after GMs degradation were studied in comparison with CPC/GMs cements. The resulting cements presented reduced setting time and increased compressive strength as the content of CSD below 5 wt%. Sustained release of GS was obtained on at least 21 days, and release rates were found to be chiefly controlled by the GMs degradation rate. After 4 weeks of degradation study, the resulting composite cements appeared macroporous, degradable and suitable compressive strength, suggesting that they have potential as controlled local drug delivery system and for cancellous bone applications.






Similar content being viewed by others
References
LeGeros R, Chohayeb A, Shulman A. Apatitic calcium phosphates: possible dental restorative materials. J Dent Res. 1982;61:343–8.
Brown W, Chow L. A new calcium phosphate setting cement. J Dent Res. 1983;62:672–8.
Urban RM, Turner TM, Hall DJ, Inoue N, Gitelis S. Increased bone formation using calcium sulfate–calcium phosphate composite graft. Clin Orthop Relat Res. 2007;459:110–7.
Ooms EM, Wolke JG, van der Waerden JP, Jansen JA. Trabecular bone response to injectable calcium phosphate (Ca–P) cement. J Biomed Mater Res. 2002;61:9–18.
Sarkar MR, Wachter N, Patka P, Kinzl L. First histological observations on the incorporation of a novel calcium phosphate bone substitute material in human cancellous bone. J Biomed Mater Res. 2001;58:329–34.
LeGeros RZ, Parsons JR, Daculsi G, Driessens F, Lee D, Liu ST, Metsger S, Peterson D, Walker M. Significance of the porosity and physical chemistry of calcium phosphate ceramics. Biodegradation–bioresorption. Ann NY Acad Sci. 1988;523:268–74.
Chow LC. Calcium phosphate cements. Monogr Oral Sci. 2001;18:148–63.
Basle MF, Chappard D, Grizon F, Filmon R, Delecrin J, Daculsi G, Rebel A. Osteoclastic resorption of Ca–P biomaterials implanted in rabbit bone. Calcif Tissue Int. 1993;53:348–56.
Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–91.
Chung HJ, Park TG. Surface engineered and drug releasing prefabricated scaffolds for tissue engineering. Adv Drug Deliv Rev. 2007;59:249–62.
Habraken WJEM, de Jonge LT, Wolke JGC, Yubao L, Mikos AG, Jansen JA. Introduction of gelatin microspheres into an injectable calcium phosphate cement. J Biomed Mater Res A. 2008;87A(3):643–55.
Habraken WJEM, Wolke JGC, Mikos AG, Jansen JA. Porcine gelatin microsphere/calcium phosphate cement composites: an in vitro degradation study. J Biomed Mater Res B. 2009;91B(2):555–61.
Li M, Liu X, Liu X, Ge B, Chen K. Creation of macroporous calcium phosphate cements as bone substitutes by using genipin-crosslinked gelatin microspheres. J Mater Sci Mater Med. 2009;20:925–34.
Clokie CML, Moghadam H, Jaskson MT, Sandor GK. Closure of critical sized defects with allogenic and alloplastic bone substitutes. J Craniofac Surg. 2002;13:111–21.
Schliephake H, Grubber R, Dard M, Wenz R, Scholz S. Repair of calvarial defects in rats by prefabricated hydroxyapatite cement implants. J Biomed Mater Res A. 2004;69:382–90.
Link DP, van den Dolder J, Jurgens WJ, Wolke JG, Jansen JA. Mechanical evaluation of implanted calcium phosphate cement incorporated with PLGA microparticles. Biomaterials. 2006;27:4941–7.
Habraken WJEM, Wolke JGC, Mikos AG, Jansen JA. Injectable PLGA microsphere/calcium phosphate cements: physical properties and degradation characteristics. J Biomater Sci Polym Ed. 2006;17(6):1057–74.
Fei Z, Hu Y, Wu D, Wu H, Lu R, Bai J, Song H. Preparation and property of a novel bone graft composite consisting of rhBMP-2 loaded PLGA microspheres and calcium phosphate cement. J Mater Sci Mater Med. 2008;19:1109–16.
Durucan C, Brown PW. Reactivity of α-tricalcium phosphate. J Mater Sci. 2002;37:963–9.
Bohner M. New hydraulic cements based on a-tricalcium phosphate–calcium sulfate dihydrate mixtures. Biomaterials. 2004;25:741–9.
Enrique F, Maria DV, Maria MG, Jose L, Ricardo T, Juan VC, Marc B. Modulation of porosity in apatitic cements by the use of α-tricalcium phosphate–calcium sulphate dihydrate mixtures. Biomaterials. 2005;26:3395–404.
Zai YJ, Cai S, Zhou W, Li XD. Mechanical behavior of calcium phosphate based cement/gelatin microspheres composite scaffolds. J Chin Ceram Soc. 2010;38(8):1568–72.
Cai S, Yao KD, Guan YH. Effects of hydroxyapatite seeds on the hydration of α-calcium phosphate cement. J Chin Ceram Soc. 2003;31(1):108–12.
Liang HC, Chang WH, Lin KJ, Sung HW. Genipin-crosslinked gelatin microspheres as a drug carrier for intramuscular administration: in vitro and in vivo studies. J Biomed Mater Res A. 2003;65:271–82.
Wei HJ, Yang HH, Chen CH, Lin WW, Chen SC, Lai PH, Chang Y, Sung HW. Gelatin microspheres encapsulated with a nonpeptide angiogenic agent, ginsenoside Rgl, for intramyocardial injection in a rat model with infarcted myocardium. J Control Release. 2007;120:27–34.
Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27:2907–15.
Beherei HH, El-Bassyouni GT, Mohamed KR. Modulation, characterization and bioactivity of new biocomposites based on apatite. Ceram Int. 2008;34:2091–7.
Zhang X, Wyss UP, Pichora D, Goosen MFA. Biodegrable controlled antibiotic release devices for osteomyelitis-optimization of release properties. J Pharm Pharmacol. 1994;46:718–24.
Frutos CP, Díez PE, Barrales-Rienda JM, Frutos G. Validation and in vitro characterization of antibiotic-loaded bone cement release. Int J Pharm. 2000;209:15–26.
Bigi A, Bracci B, Panzavolta S. Effect of added gelatin on the properties of calcium phosphate cement. Biomaterials. 2004;25:2893–9.
Shie MY, Chen DC, Wang CY, Chiang TY, Ding SJ. Immersion behavior of gelatin-containing calcium phosphate cement. Acta Biomater. 2008;4:646–55.
Guo H, Su JC, Wei J, Kong H, Liu CS. Biocompatibility and osteogenicity of degradable Ca-deficient hydroxyapatite scaffolds from calcium phosphate cement for bone tissue engineering. Acta Biomater. 2009;5:268–78.
Myung CC, Ralph DL. Calcium phosphate formation in gelatin matrix using free ion precursors of Ca2+ and phosphate ions. Dent Mater. 2009;25:261–8.
Del Real RP, Wolke JGC, Vallet-Regi M, Jansen JA. A new method to produce macropores in calcium phosphate cements. Biomaterials. 2002;23:3673–80.
Xu HHK, Simon CG Jr. Self-hardening calcium phosphate cement-mesh composite: reinforcement, macropores, and cell response. J Biomed Mater Res A. 2004;69:267–78.
Sánchez-Salcedo S, Balas F, Izquierdo-Barba I, Vallet-Regí M. In vitro structural changes in porous HA/β-TCP scaffolds in simulated body fluid. Acta Biomater. 2009;5(7):2738–51.
Xu HHK, Burguera EF, Carey LE. Strong, macroporous, and in situ-setting calcium phosphate cement-layered structures. Biomaterials. 2007;28:3786–96.
Wei J, Jia JF, Wu F, Wei SH, Zhou J, Zhang HB, Shin J, Liu CS. Hierarchically microporous/macroporous scaffold of magnesium–calcium phosphate for bone tissue regeneration. Biomaterials. 2010;31:1260–9.
Doadrio AL, Sousa EMB, Doadrio JC, Pariente JP, Barba II, Vallet-Regi M. Mesoporous SBA-15 HPLC evaluation for controlled gentamicin drug delivery. J Control Release. 2004;97:125–32.
Liu WN, Chang J. In vitro evaluation of gentamicin release from a bioactive tricalcium silicate bone cement. Mater Sci Eng C. 2009;29:2486–92.
Acknowledgments
The authors appreciate financial support from the Natural Science Foundation of China (Grant No. 50772072, 51072129, 30872639) and the Tianjin Natural Science Foundation (Grant No. 11JCYBJC02600).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, S., Zhai, Y., Xu, G. et al. Preparation and properties of calcium phosphate cements incorporated gelatin microspheres and calcium sulfate dihydrate as controlled local drug delivery system. J Mater Sci: Mater Med 22, 2487–2496 (2011). https://doi.org/10.1007/s10856-011-4432-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-011-4432-2