Abstract
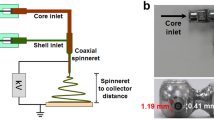
The main theme here is to fabricate PLA (poly lactic-acid)/CDHA (carbonated calcium deficient hydroxyapatite) bionanocomposites, where both the constituents are biocompatible and biodegradable with one dimension in nanometer scale. Such materials are important in tissue engineering applications. The bionanocomposite fibers were fabricated via electrospinning. There are two important signatures of this paper. First, CDHA, rather than HA, is added to PLA as the second phase. As opposed to HA, CDHA mimics the bone mineral composition better and is biodegradable. Therefore, PLA/CDHA fibers should have better biodegradability while maintaining a physiological pH during degradation. To the best of our knowledge, this is the first attempt of electrospinning of such a composite. Second, the CDHA nanoparticles were synthesized using the benign low temperature biomimetic technique, the only route available for the retention of carbonate ions in the HA lattice. The structural properties, degradation behavior, bioactivity, cell adhesion, and growth capability of as-fabricated PLA/CDHA bionanocomposites were investigated. The results show that the incorporation of CDHA decreased PLA fiber diameters, accelerated PLA degradation, buffered pH decrease caused by PLA degradation, improved the bioactivity and biocompatibility of the scaffold. These results prove that PLA/CDHA bionanocomposites have the potential in tissue regeneration applications.









Similar content being viewed by others
References
Sill TJ, von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29:1989–2006.
Roy RA, Roy R. Diphasic xerogels: I ceramic-metal composites. Mater Res Bull. 1984;19:169–77.
Roy R, Komarneni S, Roy DM. Better ceramics through chemistry. Mater Res Soc Symp Proc. 1984;32:347–59.
Komarneni S. Nanocomposites. J Mater Chem. 1992;2:1219–30.
Darder M, Aranda P, Ruiz-Hitzky E. Bionanocomposites: a new concept of ecological, bioinspired, and functional hybrid materials. Adv Mater. 2007;19:1309–19.
Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21:2335–46.
Lassalle V, Ferreira ML. PLA nano- and microparticles for drug delivery: an overview of the methods of preparation. Macromol Biosci. 2007;7:767–83.
Yamaguchi M, Shinbo T, Kanamori T, Wang PC, Niwa M, Kawakami H, Nagaoka S, Hirakawa K, Kamiya M. Surface modification of poly(l-lactic acid) affects initial cell attachment, cell morphology, and cell growth. J Artif Organs. 2004;7:187–93.
Bergsma JE, Rozema FR, Bos RRM, Boering G, de Bruijn WC. Late degradation tissue response to poly(l-lactide) bone plates and screws. Biomaterials. 1995;16:25–31.
Jeong SI, Ko EK, Yum J, Jung CH, Lee YM, Shin H. Nanofibrous poly(lactic acid)/hydroxyapatite composite scaffolds for guided tissue regeneration. Macromol Biosci. 2008;8:328–38.
McCullen SD, Zhu Y, Bernacki SH, Narayan RJ, Pourdeyhimi B, Gorga RE, Loboa EG. Electrospun composite poly(l-lactic acid)/tricalium phosphate scaffolds induce proliferation and osteogenic differentiation of human adipose-derived stem cells. Biomed Mater. 2009;4:035002.
Roeder RK, Converse GL, Kane RJ, Yue W. Hydroxyapatite-reinforced polymer biocomposites for synthetic bone substitutes. JOM. 2008;60:38–45.
Rey C. Calcium phosphate biomaterials and bone mineral. Differences in composition, structure and properties. Biomaterials. 1990;11:13–5.
Wang X, Song G, Lou T. Fabrication and characterization of nano composite scaffold of poly(l-lactic acid)/hydroxyapatite. J Mater Sic: Mater Med. 2010;21:183–8.
Kokubo T. Surface chemistry of bioactive glass-ceramics. J Non-Cryst Solids. 1990;120:138–51.
Tas AC. Synthesis of biomimetic Ca-hydroxyapatite powders at 37°C in synthetic body fluids. Biomaterials. 2000;21:1429–38.
Bigi A, Boanini E, Panzavolta S, Roveri N. Biomimetic growth of hydroxyapatite on gelatin films doped with sodium polyacrylate. Biomacromolecules. 2000;1:752–6.
Dorozhkina EI, Dorozhkin SV. Surface mineralization of hydroxyapatite in modified simulated body fluid (mSBF) with higher amounts of hydrogencarbonate ions. Colloid Surface A. 2002;210:41–8.
Oyane A, Onuma K, Ito A, Kim HM, Kokubo T, Nakamura T. Formation and growth of clusters in conventional and new kinds of simulated body fluids. J Biomed Mater Res. 2003;64:339–48.
Jalota S, Bhaduri SB, Tas AC. Effect of carbonate content and buffer type on calcium phosphonate formation in SBF solutions. J Mater Sci Mater Med. 2006;17:697–707.
Jalota S, Bhaduri SB, Tas AC. Using a synthetic body-fluid (SBF) solution of 27 mM HCO3 − to make bone substitutes more osteointegrative. Mater Sci Eng C. 2008;28:129–40.
Yin X, Stoot MJ. Biological calcium phosphates and posner’s cluster. J Chem Phys. 2003;118:3717–23.
Onuma K, Ito A. Cluster growth model for hydroxyapatite. Chem Mater. 1998;10:3346–51.
Qu H, Wei M. The effect of temperature and initial pH on biomimetic apatite coating. J Biomed Mater Res B Appl Biomater. 2008;87:204–12.
Durucan C, Brown PW. Low temperature formation of calcium-deficient hydroxyapatite-PLA/PLGA composites. J Biomed Med Res. 2000;51:717–25.
Zhang R, Ma PX. Porous poly(l-lactic acid)/apatite composites created by biomimetic process. J Biomed Mater Res. 1999;45:285–93.
Rakovsky A, Gutmanas EY, Gotman I. Ca-deficient hydroxyapatite/polylactide nanocomposites with chemically modified interfaces by high pressure consolidation at room temperature. J Mater Sci. 2010;45:6339–44.
Dorozhkin SV, Epple M. Biological and medical significance of calcium phosphates. Angew Chem Int Ed. 2002;41:3130–46.
Tylavsky FA, Spence LA, Harkness L. The importance of calcium, potassium, and acid-base homeostasis in bone health and osteoporosis prevention. J Nutri. 2008;138:164S–5S.
Takeda R, Nakamura T. Effects of high magnesium intake on bone mineral status and lipid metabolism in rats. J Nutri Sci Vitam. 2008;54:66–75.
Palazzo B, Iafisco M, Laforgia M, Margiotta N, Natile G, Bianchi CL, Walsh D. Mann Stephen, Roveri Norberto. Biomimetic hydroxyapatite-drug nanocrystals as potential bone substitutes with antitumor drug delivery properties. Adv Funct Mater. 2007;17:2180–8.
Tas AC, Bhaduri SB. Rapid coating of Ti6Al4 V at room temperature with a calcium phosphate solution similar to 10× simulated body fluid. J Mater Res. 2004;19:2742–9.
Hofmann I, Müller L, Greil P, Müller FA. Precipitation of carbonated calcium phosphate powders from a highly supersaturated simulated body fluid solution. J Am Ceram Soc. 2007;90:821–4.
Oyane A, Kim HM, Furuya T, Kokubo T, Miyazaki T, Nakamura T. Preparation and assessment of revised simulated body fluids. J Biomed Mater Res. 2003;65:188–95.
Wen HB, de Wijn JR, Cui FZ, de Groot K. Preparation of calcium phosphonate coatings on titanium implant materials by simple chemistry. J Biomed Mater Res. 1998;41:227–36.
Dorozhkin SV. Calcium orthophosphate-based biocomposites and hybrid biomaterials. J Mater Sci. 2009;44:2343–87.
Sui G, Yang X, Mei F, Hu X, Chen G, Deng X, Ryu S. Poly-l-lactic acid/hydroxyapatite hybrid membrane for bone tissue regeneration. J Biomed Mater Res A. 2007;82:445–54.
Megelski S, Stephens JS, Chase DB, Rabolt JF. Micro- and nanostructured surface morphology on electrospun polymer fibers. Macromolecules. 2002;35:8456–66.
Bognitzki M, Czado W, Frese T, Schaper A, Hellwing M, Steinhart M, Greiner A, Wenorff JH. Nanostructured fibers via electrospinning. Adv Mater. 2001;13:70–2.
Loher S, Reboul V, Brunner TJ, Simonet M, Dora C, Neuenschwander P, Stark WJ. Improved degradation and bioactivity of amorphous aerosol derived tricalcium phosphate nanoparticles in poly(lactide-co-glycolide). Nanotech. 2006;17:2054–61.
Deng XL, Sui G, Zhao ML, Chen GQ, Yang XP. Poly(l-lactic acid)/hydroxyapatite hybrid nanofibrous scaffolds prepared by electrospinning. J Biomed Sci Polymer Edn. 2007;18:117–30.
Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27:2907–15.
Acknowledgments
This work was supported by a NSF Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, H., Touny, A.H. & Bhaduri, S.B. Fabrication of novel PLA/CDHA bionanocomposite fibers for tissue engineering applications via electrospinning. J Mater Sci: Mater Med 22, 1183–1193 (2011). https://doi.org/10.1007/s10856-011-4295-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-011-4295-6