Abstract
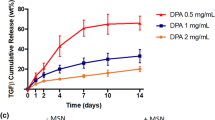
In order to induce the chondrogenesis of mesenchymal stem cells (MSCs) in tissue engineering, a variety of growth factors have been adapted and encouraging results have been demonstrated. In this study, we developed a delivery system for dual growth factors using a gelation rate controllable alginate solution (containing BMP-7) and polyion complex nanoparticles (containing TGF-β2) to be applied for the chondrogenesis of MSCs. The dual growth factors (BMP-7/TGF-β2)-loaded nanoparticle/hydrogel system showed a controlled release of both growth factors: a faster release of BMP-7 and a slower release of TGF-β2, ca., approximately 80 and 30% release at the end of an incubation period (21 days), respectively, which may be highly desirable for chondrogenic differentiation of MSCs. On the contrary, the release of each growth factor from the dual growth factors-loaded hydrogel (without the nanoparticles) was much slower than that of the nanoparticle/hydrogel system, approximately 36% (BMP-7) and 16% (TGF-β2) for 21 days, and this is more than likely attributed to the aggregation between growth factors during the hydrogel fabrication step. The nanoparticle/hydrogel system with separate growth factor loading may provide desirable growth factor delivery kinetics for cartilage regeneration, as well as the chondrogenesis of MSCs.






Similar content being viewed by others
References
Caplan AI, Goldberg VM. Principles of tissue engineered regeneration of skeletal tissues. Clin Orthop. 1999;367:S12–6.
Buckwalter JA, Mankin HJ. Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr Course Lect. 1998;47:477–86.
Beiser IH, kanat IO. Subchondral bone drilling: a treatment for cartilage defects. J foot Surg. 1990;29:595–601.
O’Driscoll SW. The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998;80:1795–812.
Gilbert JE. Current treatment options for the restoration of articular cartilage. Am J Knee Surg. 1998;11:42–6.
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–95.
Peterson L, Menche D, Grande D, Pitman M. Chondrocyte transplantation: an experimental model in the rabbit. Trans Orthop Res Soc. 1984;9:218.
Messner K, Gillquist J. Cartilage repair. A critical review. Acta Orthop Scand. 1996;67:523–9.
Brittberg M. Autologous chondrocyte transplantation. Clin Orthop. 1999;367:S147–55.
Grigolo B, Roseti L, De Franceschi L, Piacentini A, Cattini L, Manfredini M, Faccini R, Facchini A. Molecular and immunohistological characterization of human cartilage twoyears following autologous cell transplantation. J Bone Joint Surg Am. 2005;87:46–57.
Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200.
Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–50.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca DJ, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multili- neage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7.
Verfaillie CM. Adult stem cells: assessing the case for pluripotency. Trends Cell Biol. 2002;12:502–8.
Gimble JM, Guilak F. Differentiation potential of adipose derived adult stem (ADAS) cells. Curr Top Dev Biol. 2003;58:137–60.
Aust L, Devlin B, Foster SJ, Halvorsen YD, Hicok K, du Laney T, Sen A, Willingmyre GD, Gimble JM. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6:7–14.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28.
Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, Weber RM, Ewerbeck V, Richter W. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003;48:418–29.
Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–9.
Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, Guilak F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;290:763–9.
Estes BT, Wu AW, Guilak F. Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum. 2006;54:1222–32.
Hennig T, Lorenz H, Thiel A, Goetzke K, Dickhut A, Geiger F, Richter W. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGF beta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol. 2007;211:682–91.
Sheyn D, Pelled G, Zilberman Y, Talasazan F, Frank JM, Gazit D, Gazit Z. Nonvirally engineered porcine adipose tissue-derived stem cells: use in posterior spinal fusion. Stem Cells. 2008;26:1056–64.
Kim HJ, Im GI. Chondrogenic differentiation of adipose tissue-derived mesenchymal stem cells: greater doses of growth factor are necessary. J Orthop Res. 2008;27:612–9.
Kim HJ, Im GI. Combination of transforming growth factor-beta2 and bone morphogenetic protein 7 enhances chondrogenesis from adipose tissue-derived mesenchymal stem cells. Tissue Eng. 2009;15A:1543–51.
Logeart-Avramoglou D, Jozefonvicz J. Carboxymethyl benzylamide sulfonate dextrans (CMDBS), a family of biospecific polymers endowed with numerous biological properties: a review. J Biomed Mater Res. 1999;48:578–90.
Silva AKA, Richard C, Bessodes M, Scherman D, Merten OW. Growth factor deliver approaches in hydrogels. Biomacromolecular. 2009;10:9–18.
Wells LA, Sheardown H. Extended release of high pI proteins from alginate microspheres via a novel encapsulation technique. Eur J Pharm Biopharm. 2007;65:329–35.
Kierstan M, Bucke C. The immobilization of microbial cells, subcellular organelles, and enzymes in calcium alginate gels. Biotechnol Bioeng. 1977;19:387–97.
Bucke C. Cell immobilization in calcium alginate. Methods Enzymol. 1987;135:175–89.
Kanematsu A, Yamamoto S, Ozeki M, Noguchi T, Kanatani I, Ogawa O, Tabata Y. Collagenous matrices as release carriers of exogenous growth factors. Biomaterials. 2004;25:4513–20.
Jeon O, Song SJ, Kang JW, Putnam AJ, Kim BS. Enhancement of ectopic bone formation by bone morphogenetic protein-2 released from a heparin conjugated poly(L-lactic-co- glycolic acid) scaffold. Biomaterials. 2007;28:2763–71.
Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R, Hubbell JA. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21:513–8.
Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–34.
Shirai Y, Hashimoto K, Irie S. Formation of effective channels in alginate gel for immobilization of anchorage-dependent animal. Appl Microbiol Biotechnol. 1989;31:342–5.
Chung YI, Tae G, Yuk SH. A facile method to prepare heparin-functionalized nanoparticles for controlled release of growth factors. Biomaterials. 2006;27:2621–6.
Boddohi S, Moore N, Johnson PA, Kipper MJ. Polysaccharide-based polyelectrolyte complex nanoparticles from chitosan, heparin, and hyaluronan. Biomacromolecules. 2009;10:1402–9.
Hans ML, Lowman AM. Biodegradable nanoparticles for drug delivery and targeting. Curr Opin Solid St M. 2002;6:319–27.
Cho SH, Lim SM, Han DK, Yuk SH, Im GI, Lee JH. Time-dependent alginate/polyvinyl alcohol hydrogels as injectable cell carriers. J Biomater Sci Polym Edn. 2009;20:863–76.
George M, Abraham TE. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan—a review. J Control Release. 2006;114:1–14.
Andresen IL, Skipnes O, Smidsrod O, Ostgaard K, Hemmer PC. Some biological functions of matrix composition of seawater. ACS Symp Ser. 1977;48:361–81.
Nimni ME. Polypeptide growth factors: targeted delivery system. Biomaterials. 1997;18:1201–25.
Harnsilawat T, Pongsawatmanit R, McClements DJ. Characterization of β-lactoglobulin- sodium alginate interactions in aqueous solutions: a calorimetry, light scattering, electrophoretic mobility and solubility study. Food Hydrocolloids. 2006;20:577–85.
Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. Biology and clinical applications. J Bone Joint Surg Am. 2002;84A:1032–44.
Jaklenec A, Hinckfuss A, Bilgen B, Ciombor DM, Aaron R, Mathiowitz E. Biomaterials. 2008;29:1518–25.
Edelman ER, Mathiowitz E, Langer R, Klagsbrun M. Controlled and modulated release of basic fibroblast growth factor. Biomaterials. 1991;12:619–26.
Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000;65:389–402.
Lyon M, Rushton G, Gallagher JT. The interaction of the transforming growth factor-betas with heparin/heparan sulfate is isoform-specific. J Biol Chem. 1997;272:18000–6.
Acknowledgements
This work was supported by grants from the Korea Science & Engineering Foundation (Grant No. R01-2006-000-10432-0) and the Pioneer Research Center Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2010-0002176).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, S.M., Oh, S.H., Lee, H.H. et al. Dual growth factor-releasing nanoparticle/hydrogel system for cartilage tissue engineering. J Mater Sci: Mater Med 21, 2593–2600 (2010). https://doi.org/10.1007/s10856-010-4118-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-010-4118-1