Abstract
COX-2 inhibitors have demonstrated beneficial effects in colorectal cancer. The purpose of this study was to prepare and evaluate the colon specific microspheres of COX-2 inhibitors using valdecoxib as a model drug. Mucoadhesive core microspheres were prepared using chitosan as polymer and entrapped within Eudragit S 100 for colon targeting. FTIR spectrum of selected, coated microspheres showed peaks of valdecoxib at 3377, 3250, 1334 and 1155 cm−1. XRD showed amorphous character and DSC showed depressed broad endotherm of valdecoxib at 169.07°C, which may be attributed to dilution effect by the amorphous polymer. The coated microspheres were spherical with an average size of 90 μm. Storage of the microspheres at 40°C/75% relative humidity for 6 months indicated no significant drug degradation. The coated microspheres did neither release the drug in acidic pH of stomach (pH 1.2) nor in small intestinal pH between 5 to 6.8, and the release started at pH 7.4, indicting perfect colonic delivery. The coated microspheres pretreated with phosphate buffer pH 7.4 for 30 min, when applied to mucosal surface of freshly excised goat colon, showed good mucoadhesion. The drug release at pH 7.4 and good mucoadhesive property of the microspheres make the system ideal for colonic delivery.
Similar content being viewed by others
1 Introduction
Though much advances have taken place in surgery, radiation therapy and chemotherapy, cancer is still one amongst the most common causes of death in developed world, second only to heart disease [1]. Colorectal cancer, which involves the colon, rectum and the anal canal, has been reported to be the fourth most frequent cause of cancer death. The incidence rates of the disease are reported to be higher in industrialized areas such as North America, Northern and Western Europe, Australia and New Zealand, compared to the developing/less developed areas such as India, South America and rural Africa [2]. The high mortality associated with colorectal cancer demands effective prevention along with the surgery, radiation and chemotherapy. Specifically, chemoprevention approaches are more important in patients with genetic predisposition to colon cancer.
Nonsteroidal anti-inflammatory drugs (NSAIDs) have demonstrated anti carcinogenic effects in chemically induced colon cancer in animal models and their use has been reported to cause a substantial decrease in the risk of deaths from colorectal cancer [3–6]. Higher concentrations of prostaglandins (PGE2) have been detected in human tumors of colon, lung and breast compared to normal tissues [7]. Several epidemiological studies have demonstrated that the patients treated with one of the several different types of NSAIDs had a decreased risk of mortality compared to patients who did not use these routinely. On the other hand, the chronic use of NSAIDs is commonly associated with gastrointestinal bleeding and gastric ulcer. The conventional NSAIDs like aspirin, ibuprofen, diclofenac and others are reported to inhibit both the forms of cyclo-oxygenase (COX) enzyme, i.e., COX 1 and COX 2. While, COX 1 is expressed constitutively and is required for physiological processes such as maintenance of gastrointestinal mucosa and platelet aggregation, COX 2 is induced by cytokines, growth factor and mitogens [8]. Expression of COX 2 enzyme is increased up to 90% in the cases of sporadic colon carcinomas and 40% for adenomas, but not in normal colonic mucosa. Thus, it is presumed that NSAIDs mediate apoptosis via inhibition of COX 2 enzyme [9]. The dual COX inhibitory effect of the conventional NSAIDs results in gastrointestinal side effects, which restricts their use for the prevention of colorectal cancer. However, with the advent of newer COX 2 inhibitors like celecoxib, valdecoxib, rofecoxib, a new ray of hope has emerged for treatment of colorectal cancer. In 1999, the US FDA approved the use of celecoxib to reduce the number of adenomatous colorectal polyps, as an adjunct to usual care, though, some recent reports have demonstrated the cardiac toxicity of these COX-2 inhibitors [10].
The development of a suitable site specific delivery system releasing active ingredient in the colon, can lead to considerable reduction in the dose and the undue side effects of the drugs. The various approaches employed for achieving colonic drug delivery of active drugs include pro-drug approach [11, 12], time dependent formulations [13], pH dependent system [14–18] and colonic micro-flora activated system [19]. Single unit colon targeted drug delivery system like coated tablets, suffer from the disadvantage of unintentional disintegration of the formulation due to manufacturing deficiency or unusual gastric physiology that may lead to increased systemic bioavailability or loss of local action in the colon [20]. In comparison to unit drug delivery systems, development of multiparticulate dosage forms has gained momentum lately, because of their potential benefits, like increased bioavailability, reduced risk of systemic toxicity, reduced risk of local irritation and predictable gastric emptying [21]. Moreover, the smaller particle size of the multiparticulate system makes it more uniformly dispersed in the gastrointestinal tract and allows smooth passage through the GI tract [22, 23]. The anatomical difference of colonic region from that of small intestine, i.e., having less surface area (no villi) and the presence of plecal folds make the movement of colonic contents sluggish and largely propulsive. The structure and inhomogeneity of the colonic contents causes a separation according to the particle size. Consolidation starts with lumps of debris pushed ahead of the finer particles in the liquid phase. Thus, microparticulates get separated and trapped in the folds leading to increase in their retention [24]. Further the localized drug delivery can be optimized by retaining a dosage form at the site of action, which can be easily achieved by using mucoadhesive polymers in formulation of multiparticulate drug delivery system. Mucoadhesive polymers offer prolonged residence time at the site of drug absorption due to increased contact with absorbing mucous. The factors which may be expected to play important role in determining release profile of the included drug from the coated microspheres are the pH solubility of Eudragit S100 coating, the swelling and degradation behavior of core polymer, drug solubility and diffusion through the core polymer, the interaction between core and coat polymers, if any, and finally the ratio of core and coat polymers employed for the preparation of microspheres.
Taking the aforesaid information in view, valdecoxib, a COX 2 inhibitor, microspheres dispersed in chitosan (a mucoadhesive polymer) coated with Eudragit S100, a pH sensitive polymer, have been prepared. Attempts have been made to characterize the microspheres by FTIR spectroscopy, X-ray diffraction, differential scanning calorimetry and scanning electron microscopy, mucoadhesion, in vitro drug release in simulated gastrointestinal condition and stability of the formulations.
2 Materials and methods
2.1 Materials
Valdecoxib and Eudragit S100 were supplied as gift samples by Aarti Drugs Ltd. (Thane, India) and Evonik Industries (Mumbai, India), respectively. Chitosan (Brookfield viscosity > 200 cps) was purchased from Sigma–Aldrich Chemie GmbH, Riedstr, Steinheim while Span 80 were obtained from Loba Chemie Pvt. Ltd., Mumbai, India. Following ingredients were of analytical grade: liquid paraffin, glutaraldehyde solution (25%), isopropyl alcohol, potassium dihydrogen phosphate, sodium hydroxide and glacial acetic acid [Merck Specialities Pvt. Ltd., Mumbai, India], petroleum ether-boiling range 40°–60°C (RFCL Ltd. New Delhi, India), ethanol (Changshu Chemical, China), methanol (S D Fine Chem Ltd., Mumbai, India) and acetone (Qualigens, Mumbai, India).
2.2 Preparation of core valdecoxib microspheres
Core microspheres of valdecoxib in chitosan containing varying drug polymer ratio (VC-1 to VC-7) as shown in Table 1 were prepared using the following method. Known weight of valdecoxib was dispersed in varying concentration of chitosan in acetic acid. Drug polymer dispersion was then added through 22 gauge hypodermic needle into liquid paraffin (500 ml), containing 2% w/v span 80. The mixture was stirred at 3,000 rpm using a mechanical stirrer (Remi Instruments Ltd., Mumbai, India). After 5 min of complete addition of chitosan-valdecoxib dispersion to liquid paraffin, 10 ml of glutaraldehyde solution (25% v/v) was added slowly. Stirring speed was subsequently reduced to 500 rpm. Five ml of the glutaraldehyde solution was again added 1 and 2 h after the initial addition. The suspension of the microspheres in paraffin oil, thus obtained, was allowed to stand for 1 h for complete separation of the microspheres. Clear supernatant was discarded and microspheres obtained as residue were collected, washed thrice with petroleum ether to remove remnant liquid paraffin. These were kept in deep freezer at −50°C for 10 h and stored in desiccator at room temperature.
2.3 Encapsulation of core microspheres
Selected formulation of core microspheres was coated with Eudragit S100 having core: coat ratio of 1:2.5 (VCE-1) and 1:5 (VCE-2). Accordingly, core microspheres of the drug were dispersed in Eudragit S100 solution (10% w/v) in acetone-ethanol (2:1), followed by emulsification in liquid paraffin containing 2% w/v span 80, with the help of a mechanical stirrer (1,500–2,000 rpm). Stirring was continued for 3 h to facilitate the complete evaporation of the solvent. Encapsulated microspheres were filtered and washed with petroleum ether to remove liquid paraffin and dried in vacuum desiccator for 24 h.
2.4 Characterization of microspheres
2.4.1 FTIR spectroscopy
Fourier transform infrared (FTIR) spectra of selected microspheres were recorded using a FTIR spectrophotometer (Perkin Elmer model 1600-FTIR) employing KBr disc technique in the range of 4,000–400 cm−1.
2.4.2 X-ray diffraction
X-ray diffractograms of the selected microspheres were recorded using an X-ray diffractometer (X’Pert Pro, PW 3050/PW 3071; Lelyweg, The Netherlands) using nickel filtered CuKα radiation (λ = 1.540598 Å) generated at 40 kV and 30 mA and scanning rate 2°/min over a 2θ range of 10°–80°.
2.4.3 Differential scanning calorimetry
Thermal analysis of selected microspheres was performed using DSC-TA system (Perkin Elmer). All samples were sealed in a crimped aluminium pan by application of the minimum possible pressure and heated at a rate of 10°C/min from 50 to 266°C in an atmosphere of nitrogen gas by passing at a flow rate of 60 ml/min. An empty aluminium pan was utilized as the reference pan.
2.4.4 Surface morphology and particle size distribution
The shape and surface characteristics of selected microspheres were analyzed by Scanning Electron Microscope (SEM) (ZEISS EVO Series Model EVO 50). Samples mounted on aluminium stub were sputter coated with gold under reduced pressure and 30–40 nm thick gold coat was applied using BIO-RAD POLARAN sputter coater. The sample assembly was placed in microscope and vacuum was applied. The microspheres were observed under SEM at an accelerating voltage of 15 kV.
The particle size distribution of coated microspheres was determined using Particle Size Analyzer, Brookhaven Instruments Corporation, Model 90Plus. The weighed microspheres (20 mg) were suspended in double distilled water and the dispersion was examined to determine particle size distribution.
2.5 Drug loading efficiency
Accurately weighed core microspheres equivalent to 20 mg of the drug were dissolved in 100 ml ethanol and subjected to centrifugation at 3,000 rpm for 10 min. The supernatant solution was withdrawn, diluted with ethanol to have concentration ≈20 μg/ml. Absorbance of the resulting solution was measured at 244 nm in a u.v. spectrophotometer (Shimadzu UV Pharmaspec 1700), and percentage drug content in microspheres was determined.
The same procedure was adopted for measuring drug contents in the coated microspheres, using 100 mg of coated microspheres.
Following formula was employed for calculation of drug loading efficiency:
2.6 In vitro release profile
2.6.1 Core microspheres
Accurately weighed core microspheres containing 2 mg of the drug were suspended in 20 ml of 7.4 pH phosphate buffer, containing 1.5% w/v SLS. The mixture was stirred magnetically at 37°C at 50 rpm. Samples were withdrawn at specified time intervals replacing each withdrawn sample with equal volume of release medium. The withdrawn samples were centrifuged at 3,000 rpm; supernatant was filtered through 0.45 μm membrane filter, diluted to 10 ml with phosphate buffer pH 7.4 and analyzed for drug content by measuring absorbance at 244 nm. All experiments were done in triplicate.
2.6.2 Coated microspheres
Accurately weighed coated microspheres equivalent to 2 mg of drug were placed in 20 ml 0.01 N HCl (pH 2.0) and stirred magnetically at 50 rpm for 2 h. The samples were centrifuged, supernatant filtered through 0.45 μm membrane filter and analyzed for the drug content.
In a similar experiment, coated microspheres equivalent to 2 mg of drug were placed in 20 ml of phosphate buffer containing 1.5% w/v SLS and stirred magnetically at 50 rpm. The initial pH of the buffer was maintained at 5.5 for 2 h, which was increased by addition of Na2HPO4 to 6.8 and maintained for 2 h. Subsequently, the pH of the buffer was raised by further addition of Na2HPO4 to 7.4 and maintained till the completion of study. One ml of the sample was withdrawn hourly replacing each withdrawn sample with fresh release medium. The samples were centrifuged/filtered and analyzed for drug content spectrophotometrically. All experiments were run in triplicate.
2.6.3 Statistical analysis
The in vitro drug release data from microspheres were compared by statistical analysis using one way analysis of variance (ANOVA). The Student t test was performed to compare the significance of the difference between the means of two groups. P < 0.05 was considered significant.
2.7 Evaluation of in vitro mucoadhesion
The in vitro wash-off test as reported by Lehr et al. [25] was followed for the determination of extent of mucoadhesion of microspheres. The proximal large intestine of freshly slaughtered goat was cut to expose the mucosal side and washed with distilled water and phosphate buffer pH 7.4. The serosal side (2 × 2 cm) was fixed on a glass slide with the help of a thread. Coated microspheres (5 mg of VCE-2) were spread on the exposed mucosal surface, rinsed with phosphate buffer pH 7.4 and the assembly was kept in a humidity chamber (Thermotech, India, Model TH-7004) at 37°C and 90% RH, for a period of 30 min. This pretreatment was performed to dissolve the Eudragit S100 coat and to expose the core chitosan polymer. Subsequently, the complete assembly was mounted onto the tablet disintegration test apparatus (Veego, India, model VTD-AVP) with the help of a clamp and a thread. The apparatus was operated in a manner that the tissue was given regular up and down movements while immersed in the phosphate buffer pH 7.4 contained in a beaker. The time for complete wash off of microsphere from the tissue was taken as the mucoadhesion time.
2.8 Stability studies
In order to assess long term stability, three different batches of formulations for VCE-2 were subjected to accelerated stability studies as per ICH guidelines [26]. Coated microspheres were wrapped in aluminium foil laminated with polyethylene from inside. The samples were kept at 40°C ± 2°C/75% RH ± 5% in the stability chamber (Scope Enterprises, Delhi, India) for a period of 6 months. The samples were withdrawn after an interval of 15, 30, 90 and 180 days and were analyzed for drug content.
3 Results and discussions
3.1 Characterization of prepared microspheres
3.1.1 FTIR spectroscopy
FTIR spectra of the drug valdecoxib, chitosan, Eudragit S100 and the coated microspheres are presented in Fig. 1. FTIR spectrum of the pure valdecoxib showed characteristic peaks at 3377 and 3250 cm−1 due to N–H stretching of sulfonamide and at 1334 and 1150 cm−1 due to S=O stretching vibrations of sulfonamide. The spectrum of Eudragit S100 coated microspheres of valdecoxib containing chitosan showed peaks at 3377 and 3250 cm−1 due to valdecoxib, at 1734 cm−1 due to Eudragit S100 and at 1334 and 1155 cm−1 due to valdecoxib and Eudragit S100.
3.1.2 X-ray diffraction studies
X-ray diffractograms (Fig. 2) of valdecoxib indicated presence of a crystalline material having principal peaks at 22.45° and 24.35° 2θ while both the polymers chitosan and Eudragit S100 were found to be amorphous. While the diffractogram of core microspheres of valdecoxib demonstrated presence of crystalline drug embedded in the amorphous polymer, the diffractogram of coated microspheres showed an amorphous material devoid of any crystallinity. This could be attributed to dilution effect by the amorphous polymers.
3.1.3 Differential scanning calorimetry
DSC thermograms of the drug, polymers and the microspheres are presented in Fig. 3. Pure valdecoxib exhibited a melting endotherm at 174.25°C. Similarly, thermograms of the coated microspheres demonstrated a depressed, relatively broad endotherm at 169.07°C, which could be attributed due to the dilution effect of the amorphous polymers.
3.1.4 Surface morphology and particle size distribution
Scanning Electron Microscopy (SEM) of core valdecoxib microspheres in chitosan (Fig. 4) revealed mostly spherical, rough surfaced microspheres. The rough surface is indicative of the surface associated drug crystals. On the other hand, SEM of the coated microspheres of valdecoxib in chitosan, revealed mostly spherical and smooth surfaced microspheres. The average particle diameter of the coated microspheres containing chitosan was found to be 90 μm.
3.2 Drug loading efficiency
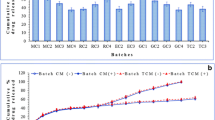
The drug loading efficiency of various formulations is presented in Table 1. Retrofit analysis of the data reveals that increasing the drug: polymer ratio from 1:5 to 1: 15 resulted in increased drug loading efficiency. However, the extent of increase was not substantial beyond the drug polymer ratio 1:10. Hence the same was kept constant and the quantity of drug and polymer were varied for compositions VC-3 TO VC-7. Composition corresponding to composition VC-7 was utilized for further studies incorporating coating with Eudragit S100 polymer in the core: coat ratio of 1:2.5 (VCE-1) and 1:5 (VCE-2). The coated microspheres revealed a loading efficiency of 92.08 and 95.39%, respectively, for VCE-1 and VCE-2.
3.3 In vitro release profile
3.3.1 Core microspheres
The in vitro release profiles of microspheres containing varying proportions of drug: chitosan as core are presented in Fig. 5. Statistical analysis of dissolution profiles of drug-chitosan microspheres (VC-3, VC-4, VC-5, VC-6 and VC-7) having core coat ratio of 1:10, but different amounts of drug and chitosan, indicated that rate and extent of drug release for VC-3 and VC-7 microspheres are the same but different from the other formulations of the microspheres. In case of microspheres containing comparatively lesser proportion of polymer, i.e., VC-5 and VC-6, there may be relatively more surface associated drug which explains the initial faster release. However, for microspheres containing more quantity of polymer, i.e., VC-4, the rate and extent of drug release is comparatively quite less as compared to all the other formulations of microspheres. However, the mean of dissolution profiles of VC-1, VC-2 and VC-3 were statistically different indicating the difference in rate and extent of drug release (P < 0.05). Thus, a change in drug: core ratio leads to modified dissolution profile, which may be attributed to the change in density of the polymer matrix and hence the diffusional path length.
3.3.2 Coated microspheres
The in vitro release profile of the coated microspheres in release medium of pH 2 indicated complete absence of any drug release even after an interval of 2 h. When the release studies were conducted in phosphate buffer (Fig. 6) and pH was gradually increased from 5.5 to 6.8, again negligible drug content in the medium was observed. However, as the pH of the medium was increased beyond 7, the drug was found to be released into the medium, which was quite expected because Eudragit S100, an enteric copolymer made of methacrylic acid-methyl methacrylate, dissolves at a pH > 7. As the release medium pH was increased to 7.4, VCE-1 microspheres showed around 20% drug release in a period of 2 h against VCE-2, which started to release drug only after 1 h residence in the release medium. Obviously, the quantity of coat was comparatively more, which took longer time to dissolve, as increased thickness of coating in tablet is known to affect oral bioavailability [27]. However, 80% of the drug was found to be released at pH 7.4 in around 4.5–5 h.
3.4 Drug release kinetics
The drug release data obtained from in vitro release experiments was subjected to various kinetics equations to evaluate the drug release mechanism and kinetics. The kinetic models used were zero order (as cumulative amount of drug released vs. time), first order (as log cumulative percentage of drug remaining vs. time [28]) and Higuchi model (as cumulative percentage of drug released vs. square root of time [29]). Moreover, Hixson-Crowell cube root law [30] was used to evaluate the drug release with changes in surface area and diameter of the particles while the mechanism of drug release was also evaluated by plotting first 60% of drug release in Korsmeyer & Peppas equation [31], as log cumulative percentage of drug released vs. log time and the exponent ‘n’ was calculated from the slope of the straight line. The release constants and regression coefficients (r 2) for all the microsphere formulations using different kinetic equations are listed in Table 2.
A thorough analysis of the table revealed that the best fit for in vitro drug release from the different chitosan based core microspheres was found to the zero order equation, indicating that the release is independent of the concentration of drug. For all these microspheres, value of ‘n’ as per K–P model was found to be between 0.45 and 0.89, which is indicative of anomalous behavior of drug release, where swelling, diffusion and erosion play an important role [32, 33]. The coated microspheres, on the other hand, demonstrated the first order release, i.e., Fickian Kinetics and value of ‘n’ (<0.45) as per K–P model also complement the same. However, this release data could also be explained by Higuchi and cube root law, though drug release from coated microspheres is believed to follow Higuchi Kinetics [34, 35].
3.5 In vitro mucoadhesive properties
Estimation of in vitro mucoadhesion revealed that the time required for complete wash off of microspheres from the mucosal tissue was 162 min, indicating good mucoadhesive properties of chitosan. Various polymer characteristics necessary for mucoadhesion have been summarized and include presence of strong hydrogen bonding groups(–OH, –COOH), strong anionic charges, high molecular weight, sufficient chain flexibility and surface free energy properties, favoring spreading onto mucus [36]. Chitosan is a cationic polymer and described as β-(1,4) 2-deoxy-2-amino d-glucan, possessing one primary amino and two free hydroxyl groups for each C6 building unit. The positive charge on primary amino group facilitates electrostatic interaction with negatively charged mucin macromolecules. This is considered to be the primary mechanism of mucoadhesion for chitosan [37]. It is quite obvious that at colonic pH (7–8), these primary amino groups get deprotonated and these deprotonated amino groups have been reported to participate in hydrogen bonding with mucin [37]. Therefore, the mucoadhesion observed at pH 7.4 can be attributed to the hydrogen bonding due to the presence of –OH groups and deprotonated –NH2 groups.
3.6 Stability studies
Selected formulation VCE-2 was subjected to accelerated stability studies as per climatic zone IV condition for assessing long term stability as per ICH protocol. After storage the formulations were analyzed for contents assay (Table 3). The content assay of the microspheres samples after the defined period indicated an insignificant difference. The results indicated that the formulation could provide a minimum shelf life of 2 years.
4 Conclusion
From the results of the present study, it can be concluded that valdecoxib microspheres prepared using chitosan as core, coated with Eudragit S100 could be used for colon targeting of drugs. Presence of chitosan in the core imparts mucoadhesion in the colon after the removal of Eudragit coat by the neutral pH of colonic contents. This eventually leads to increased residence time of the drug in the affected area. However, further in vivo studies are needed to ascertain the efficacy of the formulation in real life situation.
References
Tattersal MHN, Thomas H. Recent advances: oncology. Br Med J. 2005;318:445–8.
Pisani P, Bray F, Parkin DM. Estimates of the world wide prevalence of cancer for 25 sites in the adult population. Int J Cancer. 2002;97:72–81.
Kune S, Kune GA, Watson LF. The Melbourne Colorectal Cancer Study: incidence findings by age, sex, site, migrants and religion. Int J Epidemiol. 1986;15:483–93.
Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illnesses, operations, and medications: case control results from the Melbourne Colorectal Cancer Study. Cancer Res. 1988;48:4399–404.
Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Aspirin use and the risk for colorectal cancer and adenoma in male health professionals. Ann Intern Med. 1994;121:241–6.
Thun MJ, Namboodiri MM, Health CW Jr. Aspirin use and reduced risk of fatal colon cancer. N Eng J Med. 1991;325:1593–6.
Craven PA, Thornburg K, DeRubertis FR. Sustained increase in the proliferation of rat colonic mucosa during chronic treatment with aspirin. Gastroenterology. 1988;94:567–75.
Thun MJ, Henley SJ, Patrono C. Non steroidal anti inflammatory drugs as anticancer agents: mechanistic, pharmacologic and clinical issues. J Nat Cancer Inst. 2002;94:252–66.
Chan TA. Non steroidal anti inflammatory drugs, apoptosis and colon cancer chemoprevention. Lancet Oncol. 2002;3:166–74.
Finckh A, Aronson MD. Cardiovascular risks of cyclooxygenase-2 inhibitors: where we stand now. Ann Internal Med. 2005;142:212–4.
Bartalsky A. Salicylazobenzoic acid in ulcerative colitis. Lancet. 1982;319:960–4.
Riley SA, Turnberg LA. Sulphasalazine and aminosalicylate in the treatment of inflammatory bowl disease. Q J Med. 1990;75:561–2.
Gazzaniga A, Iamartina P, Maffione G, Sangal ME. Oral delayed release system for colonic specific delivery. Int J Pharm. 1994;108:77–83.
Ashford M, Fell J, Attwood D, Sharma H, Woodhead P. In vitro investigation into the suitability of pH dependent polymer for colonic targeting. Int J Pharm. 1993;95:193–9.
Hori M, Onishi H, Machida Y. Evaluation of Eudragit-coated chitosan microparticles as an oral immune delivery system. Int J Pharm. 2005;297:223–4.
Jain D, Panda AK, Majumdar DK. Eudragit S100 entrapped Insulin microspheres for oral delivery. AAPS Pharm Sci Tech. 2005;06:E100–7.
Marvola M, Nykanen P, Rautio S, Isonen N, Autere AM. Enteric polymer as binder and coating material in multiple unit site-specific drug delivery system. Eur J Pharm Sci. 1999;7:259–67.
Rahman Z, Kohli K, Khar RK, Ali M, Charoo NA, Shamsher AA. Characterization of 5-fluorouracil microspheres for colonic delivery. AAPS Pharm Sci Tech. 2006;7(2):E1–9.
Chourasia MK, Jain SK. Pharmaceutical approaches to colon targeted drug delivery systems. J Pharm Pharm Sci. 2003;6:33–66.
Fatima A, Asghar L, Chandran S. Multiparticulate formulation approach to colon specific drug delivery: current perspectives. J Pharm Pharm Sci. 2006;9(3):327–38.
Kramer A, Turk S, Vrecer F. Statistical optimization of diclofenac sodium sustained release pellets coated with polymethacrylic films. Int J Pharm. 2003;256:43–52.
Meyer JH, Dressman J, Fink AS, Amidon G. Effect of size and density on gastric emptying of indigestible solids. Gastroenterology. 1985;89:805–13.
Rodriguez M, Vila-Jato JL, Torres D. Design of a new multiparticulate system for potential site-specific and controlled drug delivery to the colonic region. J Control Release. 1998;55:67–77.
Rathbone MJ, Hadgraft J, Roberts MS. Modified release drug delivery technology. New York: Marcel Dekker Inc.; 2003.
Lehr CM, Bowstra JA, Tukker JJ, Junginger HE. Intestinal transit of bioadhesive microspheres in an in situ loop in the rat. J Control Release. 1990;13:51–62.
ICH Harmonised Tripartite Guideline for Stability Testing of New Drug Substances and Products.
Gibaldi M. Biopharmaceutics & clinical pharmacokinetics. 3rd ed. Phidelphia: Lea Febiger; 1984.
Wagner JG. Interpretation of percent dissolved-time plots derived from in vitro testing of conventional tablets and capsules. J Pharm Sci. 1969;58:1253–7.
Higuchi T. Mechanism of sustained action medication. J Pharm Sci. 1963;52:1145–9.
Hixson AW, Crowell JH. Dependence of reaction velocity upon surface and agitation: I—Theoretical considerations. Ind Eng Chem. 1931;23:923–31.
Bourne DW. Pharmacokinetics. In: Banker GS, Rhodes CT, editors. Modern pharmaceutics. 4th ed. New York: Marcel Dekker Inc.; 2002. p. 67–92.
Chuch HR, Zia H, Rhodes CP. Optimization of sotalol floating and bioadhesive extended release tablet formulations. Drug Dev Ind Pharm. 1995;21:1725–47.
Peppas NA. Analysis of fickian and non-fickian drug release from polymers. Pharm Acta Helv. 1985;60:110–1.
Akbuga J. Preparation and evaluation of controlled release of furosemide microspheres by spherical crystallization. Int J Pharm. 1989;53:99–105.
Jain D, Panda AK, Majumdar DK. Insulin loaded Eudragit S100 microspheres for oral delivery: preliminary in vitro studies. J Biomed Appl. 2006;21:195–211.
Peppas NA, Burim PA. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J Control Release. 1985;2:257–75.
Sogias IA, Williams AC, Khutoryanskiy VV. Why is chitosan mucoadhesive. Biomacromol. 2008;9:1837–42.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thakral, N.K., Ray, A.R. & Majumdar, D.K. Eudragit S-100 entrapped chitosan microspheres of valdecoxib for colon cancer. J Mater Sci: Mater Med 21, 2691–2699 (2010). https://doi.org/10.1007/s10856-010-4109-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-010-4109-2