Abstract
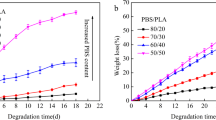
Porous polyurethane networks containing covalently attached zwitterionic compounds dihydroxypolycaprolactone phosphorylcholine and 1,2-dihydroxy-N,N-dimethylamino-propane sulfonate have been prepared and characterised. Three polymers were prepared by reacting methyl 2,6-diisocyanato hexanoate functionalised d-glucose as prepolymer A with either polycaprolactone triol alone or with addition of 10 mol% zwitterion as prepolymer B. All polymer compositions were mixed with 10 wt% hydrated gelatin beads. The cured polymers with the gelatin beads showed compression strengths that were still suitable for use in articular cartilage repair. The incorporation of zwitterions yielded more hydrophilic polymers that showed increased water absorption and increased porosity. After four months degradation in phosphate buffered saline, the polymers containing zwitterions had approximately 50% mass loss compared with 30% mass loss for that with polycaprolactone triol alone. All polymers were non-toxic in chondrocyte-based assays. Subcutaneous implantation of these polymers into rats confirmed that the polymers degraded slowly. Only a very mild inflammatory response was observed and the polymers were able to support new, well vascularised tissue formation.







Similar content being viewed by others

References
Temenoff JS, Mikos AG. Injectable biodegradable materials for orthopaedic tissue engineering. Biomaterials. 2000;21:2405–12.
Burdick JA, Frankel D, Dernell WS, Anseth KS. An initial investigation of photocurable three-dimensional lactic acid based scaffolds in a critical-sized cranial defect. Biomaterials. 2003;24:1613–20.
Ueda H, Hacker MC, Haesslein A, Jo S, Ammon DM, Borazjani RN, et al. Injectable, in situ forming poly(propylene fumarate)-based ocular drug delivery systems. J Biomed Mater Res A. 2007;83:656–66.
Muggli DS, Burkoth AK, Anseth KS. Crosslinked polyanhydrides for use in orthopaedic applications: degradation behaviour and mechanics. J Biomed Mater Res. 1999;46:271–8.
Peter SJ, Kim P, Yasko AW, Yaszemski MJ, Mikos AG. Crosslinking characteristics of an injectable poly (propylene fumarate)/beta-tricalcium phosphate paste and mechanical properties of the crosslinked composite for use as a biodegradable bone cement. J Biomed Mater Res. 1999;44:314–21.
Bonzani IC, Adhikari R, Houshyar S, Mayadunne R, Gunatillake PA, Stevens MM. Synthesis of two-component injectable polyurethanes for bone tissue engineering. Biomaterials. 2007;28:423–33.
Adhikari R, Gunatillake PA, Griffiths I, Tatai L, Wickramaratna M, Houshyar S, et al. Biodegradable injectable polyurethanes: synthesis and evaluation for orthopaedic applications. Biomaterials. 2008;29:3762–70.
Storey RF, Wiggins JS, Puckett AD. Hydrolyzable poly(ester-urethane) networks from l-lysine diisocyanate and d, l-lactide/ε-caprolactone homo- and copolymer triols. J Polym Sci A Polym Chem. 1994;32:2345–63.
Zhang JY, Beckman EJ, Piesco NP, Agarwal S. A new peptide-based urethane polymer: synthesis, biodegradation, and potential to support cell growth in vitro. Biomaterials. 2000;21:1247–58.
Chen L, Wang L, Yang ZM, Shen J, Lin SC. Synthetic studies on blood compatible biomaterials: a novel segmented polyurethane containing phosphorylcholine structure: synthesis characterization and blood compatibility evaluation. Chin J Polym Sci. 2003;21:45–50.
Yuan J, Zhu J, Zhu CH, Shen J, Lin SC. Platelet adhesion on a polyurethane surface grafted with a zwitterionic monomer of sulfobetaine via a Jeffamine spacer. Polym Intern. 2004;53:1722–8.
Glattauer V, White JF, Tsai WB, Tsai CC, Tebb TA, Danon SJ, et al. Preparation of resorbable collagen-based beads for direct use in tissue engineering and cell therapy applications. J Biomed Mater Res A. 2009; [Epub ahead of print].
Fisher RF. Propane-sultone. Indus Eng Chem. 1964;56:41–5.
Lucas HJ, Mitchell FW, Scully CN. Cyclic phosphites of some aliphatic glycols. J Am Chem Soc. 1950;72:5491–7.
Kazuhika I, Tomoko U, Nakabayshi N. Preparation of phospholipid polymers and their properties as polymer hydrogel membranes. Polym J. 1990;22:355–60.
Elvin CM, Danon SJ, Brownlee AG, White JF, Hickey M, Liyou NE, et al. Evaluation of photo-crosslinked fibrinogen as a rapid and strong tissue adhesive. J Biomed Mater Res A. 2009; [Epub ahead of print].
Franz T, Hasler EM, Hagg R, Weiler C, Jakob RP, Mainil-Varlet P. In situ compressive stiffness, biochemical composition, and structural integrity of articular cartilage of the human knee joint. Osteoarthr Cartil. 2001;9:582–92.
Oppenheimer BS, Oppenheimer ET, Danishefsky I, Stout AP, Eirich FR. Further studies of polymers as carcinogenic agents in animals. Can Res. 1955;15:333–40.
Oppenheimer BS, Oppenheimer ET, Danishefsky I, Stout AP. Carcinogenic effect of metals in rodents. Can Res. 1956;16:439–41.
Pitsner H, Gutwald R, Ordung R, Reuther J. Muhling. Poly(l-lactide): a long-term degradation study in vivo. I. Biological results. Biomaterials. 1993;14:671–7.
Acknowledgment
The authors would like to thank Professor Wei-Bor Tsai (Department of Chemical Engineering, National Taiwan University, Taiwan) for providing the gelatin beads.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adhikari, R., Danon, S.J., Bean, P. et al. Evaluation of in situ curable biodegradable polyurethanes containing zwitterion components. J Mater Sci: Mater Med 21, 1081–1089 (2010). https://doi.org/10.1007/s10856-009-3955-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-009-3955-2