Abstract
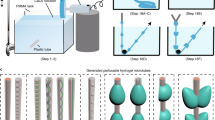
Centrifugal casting allows rapid biofabrication of tubular tissue constructs by suspending living cells in an in situ cross-linkable hydrogel. We hypothesize that introduction of laser-machined micropores into a decellularized natural scaffold will facilitate cell seeding by centrifugal casting and increase hydrogel retention, without compromising the biomechanical properties of the scaffold. Micropores with diameters of 50, 100, and 200 μm were machined at different linear densities in decellularized small intestine submucosa (SIS) planar sheets and tubular SIS scaffolds using an argon laser. The ultimate stress and ultimate strain values for SIS sheets with laser-machined micropores with diameter 50 μm and distance between holes as low as 714 μm were not significantly different from unmachined control SIS specimens. Centrifugal casting of GFP-labeled cells suspended in an in situ cross-linkable hyaluronan-based hydrogel resulted in scaffold recellularization with a high density of viable cells inside the laser-machined micropores. Perfusion tests demonstrated the retention of the cells encapsulated within the HA hydrogel in the microholes. Thus, an SIS scaffold with appropriately sized microholes can be loaded with hydrogel encapsulated cells by centrifugal casting to give a mechanically robust construct that retains the cell-seeded hydrogel, permitting rapid biofabrication of tubular tissue construct in a “bioreactor-free” fashion.






Similar content being viewed by others
References
M.S. Conte, FASEB J. 12, 43 (1998)
J.D. Kakisis, C.D. Liapis, C. Breuer, B.E. Sumpio, J. Vasc. Surg. 41, 349 (2005)
S.L. Dahl, J. Koh, V. Prabhakar, L.E. Niklason, Cell Transplant. 12, 659 (2003)
J. Hodde, Tissue Eng. 8, 295 (2002)
Q. Lu, K. Ganesan, D.T. Simionescu, N.R. Vyavahare, Biomaterials 25, 5227 (2004)
C.E. Schmidt, J.M. Baier, Biomaterials 21, 2215 (2000)
G.E. Amiel, M. Komura, O. Shapira, J.J. Yoo, S. Yazdani, J. Berry, S. Kaushal, J. Bischoff, A. Atala, S. Soker, Tissue Eng. 12, 2355 (2006)
T. Huynh, G. Abraham, J. Murray, K. Brockbank, P.O. Hagen, S. Sullivan, Nat. Biotechnol. 17, 1083 (1999)
S. Kaushal, G.E. Amiel, K.J. Guleserian, O.M. Shapira, T. Perry, F.W. Sutherland, E. Rabkin, A.M. Moran, F.J. Schoen, A. Atala, S. Soker, J. Bischoff, J.E. Mayer Jr, Nat. Med. 7, 1035 (2001)
S. Badylak, A. Liang, R. Record, R. Tullius, J. Hodde, Biomaterials 20, 2257 (1999)
G.C. Lantz, S.F. Badylak, M.C. Hiles, A.C. Coffey, L.A. Geddes, K. Kokini, G.E. Sandusky, R.J. Morff, J. Invest. Surg. 6, 297 (1993)
G.J. Wilson, D.W. Courtman, P. Klement, J.M. Lee, H. Yeger, Ann. Thorac. Surg. 60, S353 (1995)
G.J. Wilson, H. Yeger, P. Klement, J.M. Lee, D.W. Courtman, ASAIO Trans. 36, M340 (1990)
O.E. Teebken, A. Bader, G. Steinhoff, A. Haverich, Eur. J. Vasc. Endovasc. Surg. 19, 381 (2000)
A.W. Clowes, T.R. Kirkman, M.A. Reidy, Am. J. Pathol. 123, 220 (1986)
H. Bergmeister, P. Boeck, M.T. Kasimir, T. Fleck, F. Fitzal, W. Husinsky, M. Mittlboeck, H.G. Stoehr, U. Losert, E. Wolner, M. Grabenwoeger, J. Biomed. Mater. Res. B. Appl. Biomater. 74, 495 (2005)
M. Grabenwoger, F. Fitzal, J. Sider, C. Cseko, H. Bergmeister, H. Schima, W. Husinsky, P. Bock, E. Wolner, Ann. Thorac. Surg. 66, S110 (1998)
J.I. Rotmans, J.M. Heyligers, H.J. Verhagen, E. Velema, M.M. Nagtegaal, D.P. De Kleijn, F.G. De Groot, E.S. Stroes, G. Pasterkamp, Circulation 112, 12 (2005)
J.I. Rotmans, J.M. Heyligers, E.S. Stroes, G. Pasterkamp, Can. J. Cardiol. 22, 1113 (2006)
P. Roy-Chaudhury, Circulation 112, 3 (2005)
V. Mironov, V. Kasyanov, X. Zheng Shu, C. Eisenberg, L. Eisenberg, S. Gonda, T. Trusk, R.R. Markwald, G.D. Prestwich, Biomaterials 26, 7628 (2005)
X.Z. Shu, Y. Liu, Y. Luo, M.C. Roberts, G.D. Prestwich, Biomacromolecules 3, 1304 (2002)
Sharma MG (1976) J Biomech 293
C.A. Eisenberg, R.R. Markwald, Dev. Biol. 191, 167 (1997)
V. Mironov, V. Kasyanov, K. Mcallister, S. Oliver, J. Sistino, R. Markwald, J. Craniofac. Surg. 14, 340 (2003)
V. Mironov, V.A. Kasyanov, M.J. Yost, R. Visconti, W. Twal, T. Trusk, X. Wen, I. Ozolanta, A. Kadishs, G.D. Prestwich, L. Terracio, R.R. Markwald, J. Long Term Eff. Med. Implants 16, 111 (2006)
M.W. King, R.G. Guidoin, K.R. Gunasekera, C. Gosselin, Med. Prog. Technol. 9, 217 (1983)
S.T. Herbert, S.F. Badylak, L.A. Geddes, B. Hillberry, G.C. Lantz, K. Kokini, Ann. Biomed. Eng. 21, 727 (1993)
M.C. Hiles, S.F. Badylak, L.A. Geddes, K. Kokini, R.J. Morff, J. Biomed. Mater. Res. 27, 139 (1993)
M.C. Hiles, S.F. Badylak, G.C. Lantz, K. Kokini, L.A. Geddes, R.J. Morff, J. Biomed. Mater. Res. 29, 883 (1995)
V. Mironov, G.D. Prestwich, G. Forgacs, J. Mater. Chem. 17, 2054 (2007)
G.D. Prestwich, J. Cell. Biochem. 101, 1370 (2007)
G.D. Prestwich, Y. Liu, B. Yu, X.Z. Shu, A. Scott, Adv. Enzyme Regul. 47, 196 (2007)
X.Z. Shu, S. Ahmad, Y. Liu, G.D. Prestwich, J. Biomed. Mater. Res. 79, 902 (2006)
Y. Liu, X.Z. Shu, G.D. Prestwich, Tissue Eng. 12, 3405 (2006)
L. Flynn, G.D. Prestwich, J.L. Semple, K.A. Woodhouse, Biomaterials 28, 3834 (2007)
R.A. Peattie, A.P. Nayate, M.A. Firpo, J. Shelby, R.J. Fisher, G.D. Prestwich, Biomaterials 25, 2789 (2004)
R.A. Peattie, E.R. Rieke, E.M. Hewett, R.J. Fisher, X.Z. Shu, G.D. Prestwich, Biomaterials 27, 1868 (2006)
D.B. Pike, S. Cai, K.R. Pomraning, M.A. Firpo, R.J. Fisher, X.Z. Shu, G.D. Prestwich, R.A. Peattie, Biomaterials 27, 5242 (2006)
Y. Liu, S. Cai, X.Z. Shu, J. Shelby, G.D. Prestwich, Wound Repair Regen. 15, 245 (2007)
M.S. Conte, R.P. Choudhury, M. Shirakowa, J.T. Fallon, L.K. Birinyi, R.P. Choudhry, J. Vasc. Surg. 21, 413 (1995)
L.B. Kleinert, J.B. Hoying, S.K. Williams, Cell Transplant. 5, 475 (1996)
M.C. Deruiter, R.E. Poelmann, J.C. Vanmunsteren, V. Mironov, R.R. Markwald, A.C. Gittenberger-De Groot, Circ. Res. 80, 444 (1997)
Q. Shi, S. Rafii, M.H. Wu, E.S. Wijelath, C. Yu, A. Ishida, Y. Fujita, S. Kothari, R. Mohle, L.R. Sauvage, M.A. Moore, R.F. Storb, W.P. Hammond, Blood 92, 362 (1998)
E.T. Yeh, S. Zhang, H.D. Wu, M. Korbling, J.T. Willerson, Z. Estrov, Circulation 108, 2070 (2003)
Y. Liu, X.Z. Shu, G.D. Prestwich, Fertil. Steril. 87, 940 (2007)
S. Cai, Y. Liu, X.Z. Shu, G.D. Prestwich, Biomaterials 26, 6054 (2005)
K. Ghosh, X.D. Ren, X.Z. Shu, G.D. Prestwich, R.A. Clark, Tissue Eng. 12, 601 (2006)
G.D. Prestwich, X.Z. Shu, Y. Liu, S. Cai, J.F. Walsh, C.W. Hughes, S. Ahmad, K.R. Kirker, B. Yu, R.R. Orlandi, A.H. Park, S.L. Thibeault, S. Duflo, M.E. Smith, Adv. Exp. Med. Biol. 585, 125 (2006)
X.Z. Shu, K. Ghosh, Y. Liu, F.S. Palumbo, Y. Luo, R.A. Clark, G.D. Prestwich, J. Biomed. Mater. Res. 68, 365 (2004)
Acknowledgments
This research was supported by NSF FIBR Grant (EF-0526854). GDP also thanks the NIH (DC004336) and the Utah Centers of Excellence Program for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kasyanov, V.A., Hodde, J., Hiles, M.C. et al. Rapid biofabrication of tubular tissue constructs by centrifugal casting in a decellularized natural scaffold with laser-machined micropores. J Mater Sci: Mater Med 20, 329–337 (2009). https://doi.org/10.1007/s10856-008-3590-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-008-3590-3