Abstract
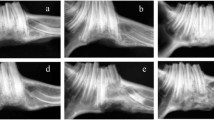
Tissue engineering techniques have been proven effective in bone regeneration and repairing load-bearing bone defects. Previous studies, however, have heretofore been limited to the use of slowdegradable or natural biomaterials as scaffolds. There are, however, no reports on using biodegradable, synthetic beta-tricalcium phosphate (β-TCP) as scaffolds to repair weight-bearing bone defects in large animals. In the present study, highly porous β-TCP scaffolds prepared by the polymeric sponge method were used to repair goat tibial defects. Fifteen goats were randomly assigned to one of three groups, and a 26 mm-long defect at the middle part of the right tibia in each goat was created. In Group A (six goats), a porous β-TCP ceramic cylinder that had been loaded with osteogenically induced autologous bone marrow stromal cells (BMSCs) was implanted in the defect of each animal. In Group B (six goats), the same β-TCP ceramic cylinder without any cells loaded was placed in the defect. In Group C (three goats), the defect was left untreated. In Group A, bony union can be observed by gross view, X-ray and micro-computed tomography (Micro-CT) detection, and histological observation at 32 weeks post-implantation. The implanted β-TCP scaffolds were almost completely replaced by tissue-engineered bone. Bone mineral density in the repaired area of Group A was significantly higher (p < 0.05) than that of Group B, in which scant new bone was formed in each defect and the β-TCP hadn’t been completely resorbed at 32 weeks. Moreover, the tissue-engineered bone of Group A had similar biomechanical properties as that of the normal left tibia in terms of bending strength and Young’s modulus (p > 0.05). In Group C, little or no new bone was formed, and non-union occurred, showing that the 26 mm segmental defect of the goat tibia was critical sized at 32 weeks. Thus, it can be concluded that the mechanical properties of the BMSCs/β-TCP composites could be much improved via tissue engineering approach and β-TCP might be used to repair the weight-bearing segmental defects of goat tibias.







Similar content being viewed by others
References
E. M. YOUNGER and M. W. CHAPMAN, J. Orthop. Trauma. 3 (1989) 192
R. C. SASSO, J. I. WILLIAMS, N. DIMASI and P. R. Jr. MEYER, J. Bone. Joint. Surg. Am. 80 (1998) 631
D. GROB, Unfallchirurg 89 (1986) 339
S. P. BRUDER, K. H. KRAUS, V. M. GOLDBERG and S. KADIYALA, J. Bone. Joint. Surg. Am. 80 (1998) 985
E. KON, A. MURAGLIA, A. CORSI, P. BIANCO, M. MARCACCI, I. MARTIN, A. BOYDE, I. RUSPANTINI, P. CHISTOLINI, M. ROCCA, R. GIARDINO, R. CANCEDDA and R. QUARTO, J. Biomed. Mater. Res. 49 (2000) 328
T. L. ARINZEH, S. J. PETER, M. P. ARCHAMBAULT, C. van den BOS, S. GORDON, K. KRAUS, A. SMITH and S. KADIYALA, J. Bone. Joint. Surg. Am. 85A (2003) 1927
L. ZHU, W. LIU, L. CUI and Y. L. CAO, Tissue. Eng. 12 (2006) 423
H. PETITE, V. VIATEAU, W. BENSAID, A. MEUNIER, C. de POLLAK, M. BOURGUIGNON, K. OUDINA, L. SEDEL and G. GUILLEMIN, Nat. Biotechnol. 18 (2000) 959
K. R. DAI, X. L. XU, T. T. TANG, Z. A. ZHU, C. F. YU, J. R. LOU and X. ZHANG, Calcif. Tissue. Int. 77 (2005) 55
M. L. JOSEPH, T. EMRE and P. G. B. MATHIAS, Clin. Orthop. 367(Suppl) (1999) 107
T. J. GAO, T. K. TUOMINEN, T. S. LINDHOLM, B. KOMMONEN and T. C. LINDHOLM, Biomaterials 18 (1997) 219
M. ROUDIER, C. BOUCHON, J. L. ROUVILLAIN, J. AMEDEE, R. BAREILLE and F. ROUAIS, J. Biomed. Mater. Res. 29 (1995) 909
K. OHURA, M. BOHNER, P. HARDOUIN, J. LEMAITRE, G. PASQUIER and B. FLAUTRE, J. Biomed. Mater. Res. 30 (1996) 193
K. OHSAWA, M. NEO, H. MATSUOKA, H. AKIYAMA, H. ITO, H. KOHNO and T. NAKAMURA, J. Biomed. Mater. Res. 52 (2000) 460
K. KURASHINA, H. KURITA, Q. WU, A. OHTSUKA and H. KOBAYASHI, Biomaterials 23 (2002) 407
M. SAITO, H. SHIMIZU, M. BEPPU and M. TAKAGI, J. Orthop. Sci. 5 (2000) 275
A. OGOSE, T. HOTTA, H. KAWASHIMA, N. KONDO, W. GU, T. KAMURA and N. ENDO, J. Biomed. Mater. Res. B. 72 (2005) 94
J. DONG, T. UEMURA, Y. SHIRASAKIE and T. TATEISHI, Biomaterials 23 (2002) 4493
M. JARCHO, Clin. Orthop. 157 (1981) 259
J. YUAN, L. CUI, W. J. ZHANG, W. LIU, and Y. L. CAO, Biomaterials 28 (2007) 1005
S. RAYNAUD, E. CHAMPION, D. BERNACHE-ASSOLIANT and P. THOMAS, Biomaterials 23 (2002) 1065
G. LIU, L. ZHAO, L. CUI, W. LIU, and Y. CAO, Biomed. Mater. 2 (2007) 78
Q. SHANG, Z. WANG, W. LIU, Y. SHI, L. CUI and Y. CAO, J. Craniofac. Surg. 12 (2001) 586
A. MURAGLIA, I. MARTIN, R. CANCEDDA and R. QUARTO, Bone 22(5 Suppl) (1998) 131S
C. MANIATOPOULOS, J. SODEK and A. H. MELCHER, Cell. Tissue. Res. 254 (1988) 317
X. L. XU, T. T. TANG, K. R. DAI, Z. A. ZHU, X. E. GUO, C. F. YU and J. R. LOU, Acta. Orthopaedica. 76 (2005) 637
K. D. JOHNSON, K. E. FRIERSON, T. S. KELLER, C. COOK, R. SCHEINBERG, J. ZERWEKH, L. MEYERS and M. F. SCIADINI, J. Orthop. Res. 14 (1996) 351
L. E. LANYON, Bone 18(1 Suppl) (1996) 37S
M. NEO, H. HERBST, C. F. VOIGT and U. M. GROSS, J. Biomed. Mater. Res. 39 (1998) 71
M. M. A. RAMSELAAR, F. C.M. DRIESSENS, W. KALK, J. R. De WIJN and P. J. Van MULLEM, J. Mater. Sci. 2 (1991) 63
M. NEO, C. F. VOIGT, H. HERBST and U. M. GROSS, J. Biomed. Mater. Res. 30 (1996) 485
J. M. SCHMITT, D. C. BUCK, S. P. JOH, S. E. LYNCH and J. O. HOLLINGER, J. Peridontol. 68 (1997) 1043
J. HANDSCHEL, H. P. WIESMANN, U. STRATMANN, J. KLEINHEINZ, U. MEYER and U. JOOS, Biomaterials 23 (2002) 1689
J. LU, M. DESCAMPS, J. DEJOU, G. KOUBI, P. HARDOUIN, J. LEMAITRE and J. P. PROUST, J. Biomed. Mater. Res. 63 (2002) 408
D. BUSER, B. HOFFMANN, J. P. BERNARD, A. LUSSI, D. METTLER and R. K. SCHENK, Clin. Oral. Implants. Res. 9 (1998) 137
Acknowledgements
This work was supported by Major State Basic Research Development Program of China (2005CB522700) and National High Technology Research and Development Program of China (2006AA02A123).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, G., Zhao, L., Zhang, W. et al. Repair of goat tibial defects with bone marrow stromal cells and β-tricalcium phosphate. J Mater Sci: Mater Med 19, 2367–2376 (2008). https://doi.org/10.1007/s10856-007-3348-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-007-3348-3