Abstract
Three different porous scaffolds were tested. The first two were prepared by sintering bovine bone. The third scaffold was prepared using three-dimensional gel-lamination, a new rapid prototyping method, and was named as hydroxyapatite artificial bone.
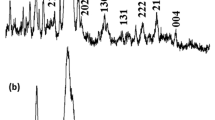
X-ray diffraction and Fourier transform infrared spectroscopy analysis confirmed that the samples were mainly highly crystalline hydroxyapatite ceramics. Scanning electron microscopy and mercury intrusion porosimetry measurement showed that the pores were interconnected and pore sizes ranged from several microns to hundreds of microns.
Mouse osteoblast-like cells grown on the three scaffolds retained their characteristic morphology. Cell proliferation and differentiation, analyzed by methylthiazol tetrazolium (MTT) and alkaline phosphatase activity assays, were significantly higher on the hydroxyapatite artificial bone than on the other two scaffolds tested. All the scaffolds provided good attachment, proliferation and differentiation of bone cells.
These results indicate that the scaffolds have a favorable interaction with cells, they support cell growth and functions, and therefore these scaffolds may have great potential as bone substitutes. The three-dimensional gel-lamination method is proven to be an attractive process to design and fabricate bone scaffolds with favorable properties, and therefore, has promising potential for bone repair applications.
Similar content being viewed by others
References
J. A. KOEMPEL and B. S. PATT J. Biomed. Mater. Res. 41 (1998) 359.
M. JARCHO, Clin. Orthop. Rel. Res. 157 (1981) 259.
H. YAMASAKI, Jpn. J. Oral. Biol. 32 (1990) 190.
U. RIPAMONTI, J. Bone. Joint. Surg. Am. 73 (1991) 692.
X. D. ZHANG, In “Bioceramics and the human body” (Amsterdam, Elsevier, 1991) p. 408.
H. YUAN and Y. LI Biomed. Eng. Appl. Basis. Com. 9 (1997) 274.
M. OKUMURA and H. OHGUSHI J. Biomed. Mater. Res. 37 (1997) 122.
C. ZHANG and J. X. WANG J. Biomed. Mater. Res. 55 (2001) 28.
M. E. NORMAN and H. M. ELGENDY Clin. Mater. 17 (1994) 85.
P. SEPULVEDA, Am. Ceram. Soc. Bull. 76 (1997) 61.
J. SAGGIO-WOYANSKY and C. E. SCOTT Am. Ceram. Soc. Bull. 71 (1992) 1674.
S. JOSCHEK and B. NIES Biomaterials. 21 (2000) 1645.
M. SIVAKUMAR and T. S. SAMPATH KUMAR Biomaterials. 17 (1996) 1709.
S. GUIZZARDI and M. RASPANTI Biomaterials. 16 (1995) 931.
X. Y. WANG and J. M. TIAN Key. Engineering. Materials. 224-2 (2002) 437.
H. SUDO and H. A. KODAMA J. Cell. Biol. 96 (1983) 191.
J. Y. CHOI and B. H. LEE J. Cell. Biochem. 61 (1996) 609.
A. G. MIKOS and M. D. LYMAN Biomaterials. 15 (1994) 55.
Y. DENG and K. ZHAO Biomaterials. 23 (2002) 4049.
F. CHEN and Z. C. WANG Mater. Lett. 57 (2002) 858.
R. N. PANDA and M. F. HSIEH J. Phys. Chem. Solids. 64 (2003) 193.
L. M. RODRÍGUEZ-LORENZO and J. M. F. FERREIRA Mater. Res. Bull. 39 (2004) 83.
J. F. OSBORN, In “Implantatwerkstoff Hydroxylapatitkeramik” (Berlin, Quintessenz Verlags-GmbH, 1985) p. 17–8; 32–6; 39.
H. YAMASAKI and H. SAKAI Biomaterials. 13 (1992) 308.
P. L. TRANQUILLI and A. MEROLLI J. Mater. Sci. Mater. Med. 5 (1994) 345.
L. CHOU and B. MAREK Biomaterials. 20 (1999) 977.
P. S. EGGLI and W. MULLER Clin. Orthop. Rel. Res. 232 (1988) 127.
R. E. HOLMES and V. MOONEY Clin. Orthop. Rel. Res. 188 (1984) 252.
W. J. DHERT and C. P. KLEIN J. Biomed. Mater. Res. 25 (1991) 1183.
A. TACHIBANA and Y. FURUTA J. Biotechnol. 93 (2002) 165.
E. WINTERMANTEL and J. MAYER Biomaterials. 17 (1996) 83.
E. TSURUGA and H. TAKITA J. Biochem (Tokyo). 121 (1997) 317.
L. L. HENCH, J. Am. Ceram. Soc. 74 (1991) 1487.
F. B. BAGAMBISA and U. JOOS J. Biomed. Mater. Res. 27 (1993) 1047.
C. P. KLEIN and A. A. DRIESSEN J. Biomed. Mater. Res. 17 (1983) 769.
P. SEPULVEDA and F. S. ORTEGA J. Am. Ceram. Soc. 83 (2000) 3021.
L. J. GIBSON and M. F. ASHBY, In “Cellular solids: structure and properties. Cambridge solid state science series, 2 nd Ed” (Cambridge, UK, Cambridge University Press, 1997) p. 429.
W. C. VROUWENVELDER and C. G. GROOT Biomaterials. 13 (1992) 382.
P. J. MARIE, Calcif. Tissue. Int. 56 Suppl 1 (1995) S13.
D. A. PULEO and L. A. HOLLERAN J. Biomed. Mater. Res. 25 (1991) 711.
M. HOTT and B. NOEL J. Biomed. Mater. Res. 37 (1997) 508.
H. ZREIQAT and P. EVANS J. Biomed. Mater. Res. 44 (1999) 389.
D. D. DELIGIANNI and N. D. KATSALA Biomaterials. 22 (2001) 87.
K. HATANO and H. INOUE Bone. 25 (1999) 439.
J. Y. MARTIN and Z. SCHWARTZ J. Biomed. Mater. Res. 29 (1995) 389.
J. C. DUBOIS and C. SOUCHIER Biomaterials. 20 (1999) 1841.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, Y., Cao, WL., Wang, XY. et al. Characterization and osteoblast-like cell compatibility of porous scaffolds: bovine hydroxyapatite and novel hydroxyapatite artificial bone. J Mater Sci: Mater Med 17, 815–823 (2006). https://doi.org/10.1007/s10856-006-9840-3
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10856-006-9840-3