Abstract
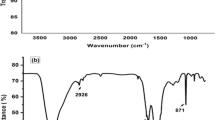
A Schiff base (E)-2((3-methylbenzilidene) amino) phenol (EMBAP)/multi-walled carbon nanotube modified carbon paste electrode was applied to the selective and accurate determination of Hg2+ and Pb2+ ions by voltammetric methods. The morphology of electrodes surface was characterized, and a density functional theory (DFT) based on the interaction of target ions with EMBAP was investigated. The theoretical binding energies, entropies, free energies and formation constants were calculated. The results indicated that complexes are stable, and formation is spontaneous. The synergistic advantages of EMBAP and MWNTs caused higher sensitivity, fast electron transfer, well reproducibility, long time stability, and well anti-interference capability. The theoretical studies showed that the most important interaction is the electron-donating O lone pair to the contacting LP* of metal ions. Under optimum conditions, the peak current was linear in 0.1–200 μmol L−1 and 0.1–250 μmol L−1 concentration of Hg2+ and Pb2+ with limits of detection (S/N = 3) of 28 nmol L−1 and 41 nmol L−1 for Hg2+ and Pb2+, respectively. The current method was applied for the simultaneous determination of Hg2+ and Pb2+ in algae samples with comparable accuracy and precision respect to reference methods.






Similar content being viewed by others
References
A. Jordanova, A. Strezov, M. Ayranov, N. Petkov, T. Stoilova, Water Sci. Technol. 39, 207–212 (1999)
E. Sandau, P. Sandau, Acta Biotechnol. 16, 227–235 (1996)
M.A. Tarighat, Food Chem. 192, 548–556 (2016)
M.A. Tarighat, K. Mohammadi, Environ. Monit. Assess. 187, 197 (2015)
D. Fenga, C. Aldrich, Hydrometallurgy 73, 1–10 (2004)
M. Ghaedi, M. Reza Fathi, A. Shokrollahi, F. Shajarat, Anal. Lett. 39, 1171–1185 (2006)
L. Rahman, W. Corns, D. Bryce, P. Stockwell, Talanta 52, 833–843 (2000)
C. Huang, B. Hu, Spectrochim Acta B 63, 437–444 (2008)
Y. Guo, Z. Wang, W. Qu, H. Shao, X. Jiang, Biosens. Bioelectron. 26, 4064–4069 (2011)
A.V. Pawar, S.S. Kanapally, K.D. Kadam, S.L. Patil, V.S. Dongle, S.A. Jadhav, S. Kim, T.D. Dongale, J. Mater. Sci. Mater. Electron. 30, 11383–11394 (2019)
D.N. Kumar, J. Roy, S.A. Alex, N. Chandrasekaran, A. Mukherjee, RSC Adv. 6, 21261–21270 (2016)
A. Afkhami, H. Ghaedi, T. Madrakian, M. Rezaeivala, Electrochim. Acta 89, 377–386 (2013)
M. Ghanei-Motlagh, M.A. Taher, A. Heydari, R. Ghanei-Motlagh, V.K. Gupta, Mater. Eng. Sci. C 63, 367–375 (2016)
Y. Wei, C. Gao, F.L. Meng, H.H. Li, L. Wang, J.H. Liu, X. Huang, J. Phys. Chem. C 116, 1034–1041 (2011)
D. Yang, L. Wang, Z. Chen, M. Megharaj, R. Naidu, Electrochim. Acta 132, 223–229 (2014)
A. Afkhami, H. Bagheri, H. Khoshsafar, M. Saber-Tehrani, M. Tabatabaee, A. Shirzadmehr, Anal. Chim. Acta 746, 98–106 (2012)
A. Kareem, H. Zafar, A. Sherwani, M. Owais, T.A. Khan, J. Mol. Struct. 1075, 17–25 (2014)
E. Pahontu, D.C. Ilies, S. Shova, C. Paraschivescu, M. Badea, A. Gulea, T. Rosu, Molecules 20, 5771–5792 (2015)
S.K. Bharti, G. Nath, R. Tilak, S.K. Singh, Eur. J. Med. Chem. 45, 651–660 (2010)
A. Afkhami, F. Soltani-Felehgari, T. Madrakian, H. Ghaedi, M. Rezaeivala, Anal. Chim. Acta 771, 21–30 (2013)
C.J. Cramer, D.G. Truhlar, Phys. Chem. Chem. Phys. 11(2009), 10757–10816 (2009)
T. Karami, New Schiff bases compounds derived from benzaldeyde derivatives and 2-aminophenol: Synthesis, characterization and thermodynamics, MSc Thesis, Chemistry Department, Faculty of Sciences, Persian Gulf University Bushehr, 2014
A.I. Tamayo, A.M. Guas, J.J. Leyte-Vidal, M. Maccini, J. Electrochem. Sci. Eng. 61, 45–54 (2014)
D. Dastan, S.L. Panahi, A.P. Yengantiwar, A.G. Banpurkar, Adv. Sci. Lett. 22, 950–953 (2016)
D. Dastan, N. Chaure, M. Kartha, J. Mater. Sci. 28, 7784–7796 (2017)
D. Dastan, A.G. Banpurkar, J. Mater. Sci. 28, 3851–3859 (2016)
J. Li, H. Xie, L. Chen, Sens. Actuator B 153, 239–245 (2011)
F.S. Zhang, J.O. Nriagu, H. Itoh, Water Res. 39, 389–395 (2005)
Y. Shi, H. Wang, X. Jiang, B. Sun, B. Song, Y. Su, Y. He, Anal. Chem. 88, 3723–3729 (2016)
M.B. Gumpu, M. Veerapandian, U.M. Krishnan, J.B.B. Rayappan, Talanta 162, 574–582 (2017)
Y. Zhang, H. Zhao, Z. Wu, Y. Xue, X. Zhang, Y. He, Z. Yuan, Biosens. Bioelectron. 48, 180–187 (2013)
M. Ghanei-Motlagh, M.A. Taher, A. Heydari, R. Ghanei-Motlagh, V.K. Gupta, Mater. Eng. Sci. C 63, 367–375 (2016)
M. Ghiaci, B. Rezaei, R.J. Kalbasi, Talanta 73, 37–45 (2007)
A. Afkhami, F. Soltani-Felehgari, T. Madrakian, H. Ghaedi, M. Rezaeivala, Anal. Chim. Acta 771, 21–30 (2013)
R.Y. Hassan, M.S. Kamel, H.N. Hassan, E. Khaled, J. Electroanal. Chem. 75, 101–106 (2015)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rezaei, A., Abbasi Tarighat, M. & Mohammadi, K. Characterization and theoretical studies of synthesized (E)-2((3-methylbenzilidene) amino) phenol complexes for the fabrication of novel electrochemical sensor for determination of Pb2+ and Hg2+ ions. J Mater Sci: Mater Electron 30, 13347–13359 (2019). https://doi.org/10.1007/s10854-019-01702-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-01702-5