Abstract
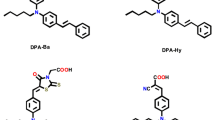
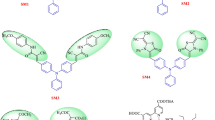
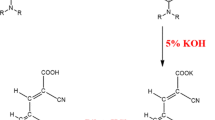
Series of metal-free dyes (OMS4-7) featuring a dibenzofulvene connected with thienyl or furanyl as conjugation bridge, T-shaped diarylamines (diphenylamine or phenyl-1-naphthylamine) as donors and different numbers of cyanoacrylic acid as anchors have been synthesized and applied into dye-sensitized solar cells (DSSCs). The efects of different donors, conjuation segments and number of anchors on optical and photovoltaic properties were investigated through photophysics, electrochemical and photovoltaic measurements. Of the DSSCs, the structure contains mono anchor with thienyl dibenzofulvene linked to diphenylamine, i.e. OMS4-based device exhibited the highest power conversion efficiency (PCE) of 2.42%, a value of Jsc of 6.01 mA cm−2, a Voc of 0.63 V, and a fill factor of 0.63 under 10 mM CDA co-adsorbent and AM 1.5 irradiation. The corresponding device was also conducted with respect to different light intensity such as D65, CWF and TL84. As a result, the cell performance based on TL84 with 2500 lx exhibited the best PCE of 8.78%.










Similar content being viewed by others
References
B. O’Reagen, M. Grätzel, A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353, 737–740 (1991)
M. Grätzel, Recent advances in sensitized mesoscopic solar cells. Acc. Chem. Res. 42, 1788–1798 (2009)
M.B. Desta, N.S. Vinh, C.H.P. Kumar, S. Chaurasia, W.T. Wu, J.T. Lin, T.C. Wei, E.W.G. Diau, Pyrazine-incorporating panchromatic sensitizers for dye sensitized solar cells under one sun and dim light. J. Mater. Chem. A 6, 13778–13789 (2018)
K.S.K. Reddy, Y.C. Liu, H.H. Chou, K. Kala, T.C. Wei, C.Y. Yeh, Synthesis and characterization of novel β-bis(N, N-diarylamino)-substituted porphyrin for dye-sensitized solar cells under 1 sun and dim light conditions. ACS Appl. Mater. Interfaces. 10, 39970–39982 (2018)
A. Hagfeldt, G. Boschloo, L.C. Sun, L. Kloo, H. Pettersson, Dye-sensitized solar cells. Chem. Rev. 110, 6595–6663 (2010)
C.Y. Chen, M. Wang, J.Y. Li, N. Pootrakulchote, L. Alibabaei, C.H. Ngoc-Le, J.D. Decoppet, J.H. Tsai, C. Grätzel, C.G. Wu, S.M. Zakeeruddin, M. Grätzel, Highly efficient light-harvesting ruthenium sensitizer for thin-film dye-sensitized solar cells. ACS Nano 3, 3103–3109 (2009)
W.C. Chen, F.T. Kong, Z.Q. Li, J.H. Pan, X.P. Liu, F.L. Guo, L. Zhou, Y. Huang, T. Yu, S.Y. Dai, Superior light-harvesting heteroleptic ruthenium (II) complexes with electron-donating antennas for high performance dye-sensitized solar cells. ACS Appl. Mater. Interfaces. 8, 19410–19417 (2016)
M. Urbani, M. Grätzel, M.K. Nazeeruddin, T. Torres, Meso-substituted porphyrins for dye-sensitized solar cells. Chem. Rev. 114, 12330–12396 (2014)
K. Kakiage, Y. Aoyama, T. Yano, K. Oya, J.I. Fujisawa, M. Hanaya, Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 51, 15894–15897 (2015)
Y.K. Eom, I.T. Choi, S.H. Kang, J. Lee, J. Kim, M.J. Ju, H.K. Kim, Thieno[3,2‐b][1]benzothiophene derivative as a new π‐bridge unit in D–π–A structural organic sensitizers with over 10.47% efficiency for dye‐sensitized solar cells. Adv. Energy Mater. 5, 1500300 (2015)
Z. Yao, H. Wu, Y. Li, J. Wang, J. Zhang, M. Zhang, Y. Guo, P. Wang, Dithienopicenocarbazole as the kernel module of low-energy-gap organic dyes for efficient conversion of sunlight to electricity. Energy Environ. Sci. 8, 3192–3197 (2015)
A. Mishra, M.K.R. Fischer, P. Bäuerle, Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules. Angew. Chem. Int. Ed. 48, 2474–2499 (2009)
Y.S. Yen, H.H. Chou, Y.C. Chen, C.Y. Hsu, J.T. Lin, Recent developments in molecule-based organic materials for dye-sensitized solar cells. J. Mater. Chem. 22, 8734–8747 (2012)
S. Ahmad, E. Guillén, L. Kavan, M. Grätzel, M.K. Nazeeruddin, Metal free sensitizer and catalyst for dye sensitized solar cells. Energy Environ. Sci. 6, 3439–3466 (2013)
N. Manfredi, B. Cecconi, A. Abbotto, Multi-branched multi-anchoring metal-free dyes for dye-sensitized solar cells. Eur. J. Org. Chem. 2014, 7069–7086 (2014)
Y.C. Chen, J.T. Lin, Multi-anchored sensitizers for dye-sensitized solar cells. Sustain. Energy Fuels 1, 969–985 (2017)
A. Peddapuram, H. Cheema, R.E. Adams, R.H. Schmehl, J.H. Delcamp, A stable panchromatic green dual acceptor, dual donor organic dye for dye-sensitized solar cells. J. Phys. Chem. C 121, 8770–8780 (2017)
H. Meier, Z.S. Huang, D. Cao, Double D–π–A branched dyes – a new class of metal-free organic dyes for efficient dye-sensitized solar cells. J. Mater. Chem. C 5, 9828–9837 (2017)
W. Li, Y. Wu, X. Li, Y. Xie, W. Zhu, Absorption and photovoltaic properties of organic solar cell sensitizers containing fluorene unit as conjunction bridge. Energy Environ. Sci. 4, 1830–1837 (2011)
S. Chaurasia, Y.C. Chen, H.H. Chou, Y.S. Wen, J.T. Lin, Coplanar indenofluorene-based organic dyes for dye-sensitized solar cells. Tetrahedron 68, 7755–7762 (2012)
Y. Bai, J. Zhang, D. Zhou, Y. Wang, M. Zhang, P. Wang, Engineering organic sensitizers for iodine-free dye-sensitized solar cells: red-shifted current response concomitant with attenuated charge recombination. J. Am. Chem. Soc. 133, 11442–11445 (2011)
C. Du, C. Li, W. Li, X. Chen, Z. Bo, C. Veit, Z. Ma, U. Wuerfel, H. Zhu, W. Hu, F. Zhang, 9-Alkylidene-9H-fluorene-containing polymer for high-efficiency polymer solar cells. Macromolecules 44, 7617–7624 (2011)
H. Li, R. Tang, Y. Hou, Y. Yang, J. Chen, L. Liu, H. Han, T. Peng, Q. Li, Z. Li, Diphenyldibenzofulvene-based sensitizers for efficient dye-sensitized solar cells: the tuned absorption properties and partially suppressed aggregation. Asian J. Org. Chem. 3, 176–184 (2014)
A.L. Capodilupo, L. De Marco, E. Fabiano, R. Giannuzzi, A. Scrascia, C. Clarlucci, G.A. Corrente, M.P. Cipolla, G. Gigli, G. Ciccarella, New organic dyes based on a dibenzofulvene bridge for highly efficient dye-sensitized solar cells. J. Mater. Chem. A 2, 14181–14188 (2014)
A.L. Capodilupo, L. De Marco, G.A. Corrente, R. Giannuzzi, E. Fabiano, A. Cardone, G. Gigli, G. Ciccarella, Synthesis and characterization of a new series of dibenzofulvene based organic dyes for DSSCs. Dyes Pigments 13, 79–89 (2016)
A.L. Capodilupo, R. Giannuzzi, G.A. Corrente, L. De Marco, E. Fabiano, A. Cardone, G. Gigli, G. Ciccarella, Synthesis and photovoltaic performance of dibenzofulvene-based organic sensitizers for DSSC. Tetrahedron 72, 5788–5797 (2016)
G.A. Corrente, E. Fabiano, L. De Marco, G. Accorsi, R. Giannuzzi, A. Cardone, G. Gigli, G. Ciccarella, A.L. Capodilupo, Effects of donor position on dibenzofulvene-based organic dyes for photovoltaics. J. Mater. Sci.: Mater. Electron. 28(12), 8694–8707 (2017)
J.T. Lin, P.C. Chen, Y.S. Yen, Y.C. Hsu, H.H. Chou, M.C.P. Yeh, Organic dyes containing furan moiety for high-performance dye-sensitized solar cells. Org. Lett. 11, 97–100 (2009)
O. Gidron, M. Bendikov, α-Oligofurans: an emerging class of conjugated oligomers for organic electronics. Angew. Chem. Int. Ed. 53, 2546–2555 (2014)
W. Jiang, Y. Sun, X. Wang, Q. Wang, W. Xu, Synthesis and photochemical properties of novel 4-diarylamine-1,8-naphthalimide derivatives. Dyes Pigments 77, 125–128 (2008)
R.Y.Y. Lin, F.L. Wu, C.H. Chang, H.H. Chou, T.M. Chuang, T.C. Chu, C.Y. Hsu, P.W. Chen, K.C. Ho, Y.H. Lo, J.T. Lin, Y-shaped metal-free D–π–(A)2 sensitizers for high-performance dye-sensitized solar cells. J. Mater. Chem. A 2, 3092–3101 (2014)
Z.S. Wang, H. Kawauchi, T. Kashima, H. Arakawa, Significant influence of TiO2 photoelectrode morphology on the energy conversion efficiency of N719 dye-sensitized solar cell. Coord. Chem. Rev. 248, 1381–1389 (2004)
M. Nomura, K. Fukukawa, Y. Shibasaki, M. Ueda, New amorphous hole-transporting molecular materials: 1,1,1-Tris(4-(4-diarylaminobenzoyloxy)phenyl)ethane. Synth. Met. 132, 9–13 (2002)
A. Baheti, K.R.J. Thomas, C.P. Lee, K.C. Ho, Synthesis and characterization of dianchoring organic dyes containing 2, 7-diaminofluorene donors as efficient sensitizers for dye-sensitized solar cells. Org. Electron. 14, 3267–3276 (2013)
Y. Shao, L.F. Molnar, Y. Jung, J. Kussmann, C. Ochsenfeld, S.T. Brown, A.T. Gilbert, L.V. Slipchenko, S.V. Levchenko, D.P. O’Neill, R.A. DiStasio Jr., Advances in methods and algorithms in a modern quantum chemistry program package. Phys. Chem. Chem. Phys. 8(27), 3172–3191 (2006)
Acknowledgements
Financial support from the Ministry of Science and Technology (Grant Nos. MOST 106-2221-E-151-046 -MY2 and MOST 106-2113-M-029-001) Taiwan ROC is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, YC., Huang, GW., Chang, YJ. et al. Branched dibenzofulvene-based organic dyes for dye-sensitized solar cells under one sun and dim light. J Mater Sci: Mater Electron 30, 12981–12991 (2019). https://doi.org/10.1007/s10854-019-01660-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-01660-y