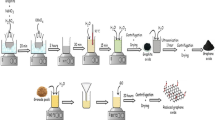
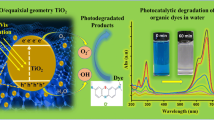
Abstract
The increase in photocatalytic activity of reduced graphene oxide–TiO2 heterostructures under ultraviolet and visible illumination is already well known, as the photocatalyst mechanism modifications with heterostructure formation. However, which step in the degradation mechanism is modified with reduced graphene oxide–TiO2 heterostructure formation has been not demonstrated yet. These specific modifications are caused by the alteration in reactive oxygen species production. In this way, the goal of this study is defined which reactive oxygen species are produced by reduced graphene oxide–TiO2 heterostructure in the photocatalytic mechanism. A fast synthesis method to obtain this heterostructure by the microwave-assisted solvothermal method is presented, obtaining an improvement of photocatalytic efficiency, under UV and visible illumination. The non-hydrolytic method favors a better distribution of TiO2 nanoparticles around the reduced graphene oxide structure and inhabits the charge carrier recombination, showing a faster electron transfer than TiO2 samples. The RhB discoloration mechanism confirms that the reduced graphene oxide presence modifies the main reactive oxygen species produced. Under UV illumination, O2H* radical is the dominant reactive oxygen species produced by TiO2. For the heterostructure, the direct oxidation by oxygen vacancy is the primary mechanism step. Under visible illumination, O2H* is the main reactive oxygen species for both materials. The results present a better understanding of principal reasons related to the improvement in photocatalytic activity and could be useful in semiconductor heterostructure design.










Similar content being viewed by others
Change history
23 August 2018
The original version of this article contains a missed citation in the Material and Methods Section, GO synthesis.
References
F. Pei, S. Xu, W. Zuo et al., Effective improvement of photocatalytic hydrogen evolution via a facile in-situ solvothermal N-doping strategy in N-TiO2/N-graphene nanocomposite. Int. J. Hydrog. Energy 39, 6845–6852 (2014). https://doi.org/10.1016/j.ijhydene.2014.02.173
P. Fernández-Ibáñez, M.I. Polo-López, S. Malato et al., Solar photocatalytic disinfection of water using titanium dioxide graphene composites. Chem. Eng. J. 261, 36–44 (2014). https://doi.org/10.1016/j.cej.2014.06.089
S. Huang, Z. Si, X. Li et al., A novel Au/r-GO/TNTs electrode for H2O2, O2 and nitrite detection. Sen. Actuators B 234, 264–272 (2016). https://doi.org/10.1016/j.snb.2016.04.167
Q. Xiang, B. Cheng, J. Yu, Graphene-based photocatalysts for solar-fuel generation. Angew Chem. Int. Ed. (2015). https://doi.org/10.1002/anie.201411096
Q. Xiang, J. Yu, M. Jaroniec, Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 41, 782 (2012). https://doi.org/10.1039/c1cs15172j
J. Liu, H. Bai, Y. Wang et al., Self-assembling TiO2 nanorods on large graphene oxide sheets at a two-phase interface and their anti-recombination in photocatalytic applications. Adv. Funct. Mater. 20, 4175–4181 (2010). https://doi.org/10.1002/adfm.201001391
A.H. Cheshme Khavar, G. Moussavi, A.R. Mahjoub, The preparation of TiO2@rGO nanocomposite efficiently activated with UVA/LED and H2O2 for high rate oxidation of acetaminophen: catalyst characterization and acetaminophen degradation and mineralization. Appl. Surf. Sci. 440, 963–973 (2018). https://doi.org/10.1016/j.apsusc.2018.01.238
N. Sun, J. Ma, C. Wang et al., A facile and efficient method to directly synthesize TiO2/rGO with enhanced photocatalytic performance. Superlatt. Microstruct. 121, 1–8 (2018). https://doi.org/10.1016/J.SPMI.2018.07.017
M. Dhanasekar, V. Jenefer, R.B. Nambiar et al., Ambient light antimicrobial activity of reduced graphene oxide supported metal doped TiO2 nanoparticles and their PVA based polymer nanocomposite films. Mater. Res. Bull. 97, 238–243 (2018). https://doi.org/10.1016/j.materresbull.2017.08.056
A.V.F.M.V. Ramana, R.S.T. Jadhav, G.S.G. Dae, Y. Kim, TiO2/reduced graphene oxide composite based nano-petals for supercapacitor application: effect of substrate. J. Mater. Sci. (2018). https://doi.org/10.1007/s10854-018-9146-5
B. Chai, J. Li, Q. Xu, K. Dai, Facile synthesis of reduced graphene oxide/WO3 nanoplates composites with enhanced photocatalytic activity. Mater. Lett. 120, 177–181 (2014). https://doi.org/10.1016/j.matlet.2014.01.094
M. Long, Y. Qin, C. Chen et al., Origin of visible light photoactivity of reduced graphene oxide/TiO2 by in situ hydrothermal growth of undergrown TiO2 with Graphene Oxide. J. Phys. Chem. C 117, 16734–16741 (2013)
M. Andreozzi, M.G. Álvarez, S. Contreras et al., Treatment of saline produced water through photocatalysis using rGO–TiO2 nanocomposites. Catal Today (2018). https://doi.org/10.1016/j.cattod.2018.04.048
Y. Yuan, Y. Leigh, A. Xuchuan, Experimental and theoretical studies of gold nanoparticle decorated zinc oxide nanoflakes with exposed {10 0} facets for butylamine sensing. Sens. Actuators B (2016). https://doi.org/10.1016/j.snb.2016.02.091
D. Chen, H. Zhang, S. Hu, J. Li, Preparation and enhanced photoelectrochemical performance of coupled bicomponent ZnO–TiO2 nanocomposites. J. Phys. Chem. C 112, 117–122 (2008). https://doi.org/10.1021/jp077236a
A. Habib, T. Shahadat, N.M. Bahadur et al., Synthesis and characterization of ZnO–TiO2 nanocomposites and their application as photocatalysts. Int. Nano Lett. 3, 1–8 (2013). https://doi.org/10.1186/2228-5326-3-5
A.K. Geim, K.S.S. Novoselov, The rise of graphene. Nat. Mater. 6, 183–191 (2007). https://doi.org/10.1038/nmat1849
A.K. Geim, Graphene: status and prospects. Science 324, 1530–1534 (2009). https://doi.org/10.1126/science.1158877
D.P. Volanti, D. Keyson, J.A. Varela, E. Longo, Aparato assistido por microondas para síntese hidrotérmica de óxidos nanoestruturados. Universidade Estadual Paulista And Universidade Federal de São Carlos (Brasil). Br N. Pi0815393-0 07 Dez. 2010 (2008)
G.B. Soares, B. Bravin, C.M.P. Vaz, C. Ribeiro, Facile synthesis of N-doped TiO2 nanoparticles by a modified polymeric precursor method and its photocatalytic properties. Appl. Catal. B 106, 287–294 (2011). https://doi.org/10.1016/j.apcatb.2011.05.018
M. Dawson, G.B. Soares, C. Ribeiro, Influence of calcination parameters on the synthesis of N-doped TiO2 by the polymeric precursors method. J. Solid State Chem. 215, 211–218 (2014). https://doi.org/10.1016/j.jssc.2014.03.044
S.A. Bakar, G. Byzynski, C. Ribeiro, Synergistic effect on the photocatalytic activity of N-doped TiO2 nanorods synthesised by novel route with exposed (110) facet. J. Alloys Compd. 666, 38–49 (2016). https://doi.org/10.1016/j.jallcom.2016.01.112
H. Wang, H. Gao, M. Chen et al., Microwave-assisted synthesis of reduced graphene oxide/titania nanocomposites as an adsorbent for methylene blue adsorption. Appl. Surf. Sci. 360, 840–848 (2016). https://doi.org/10.1016/j.apsusc.2015.11.075
F. Liu, X. Yan, X. Chen et al., Mesoporous TiO2 nanoparticles terminated with carbonate-like groups: amorphous/crystalline structure and visible-light photocatalytic activity. Catal. Today 264, 243–249 (2016). https://doi.org/10.1016/j.cattod.2015.07.012
K.H. Leong, L.C. Sim, D. Bahnemann et al., Reduced graphene oxide and Ag wrapped TiO2 photocatalyst for enhanced visible light photocatalysis. Appl. Mater. 3, 104503 (2015). https://doi.org/10.1063/1.4926454
M.Q. Yang, Y.J. Xu, Basic principles for observing the photosensitizer role of graphene in the graphene-semiconductor composite photocatalyst from a case study on graphene-ZnO. J. Phys. Chem. C 117, 21724–21734 (2013). https://doi.org/10.1021/jp408400c
J. Qin, X. Zhang, C. Yang et al., ZnO microspheres-reduced graphene oxide nanocomposite for photocatalytic degradation of methylene blue dye. Appl. Surf. Sci. (2016). https://doi.org/10.1016/j.apsusc.2016.09.043
Y. Zhang, C. Xie, F.L. Gu et al., Significant visible-light photocatalytic enhancement in Rhodamine B degradation of silver orthophosphate via the hybridization of N-doped graphene and poly(3-hexylthiophene). J. Hazard. Mater. 315, 23–34 (2016). https://doi.org/10.1016/j.jhazmat.2016.04.068
L.-L. Tan, W.-J. Ong, S.-P. Chai et al., Visible-light-active oxygen-rich TiO2 decorated 2D graphene oxide with enhanced photocatalytic activity toward carbon dioxide reduction. Appl. Catal. B 179, 160–170 (2015). https://doi.org/10.1016/j.apcatb.2015.05.024
A. Ambrosi, M. Pumera, Electrochemically exfoliated graphene and graphene oxide for energy storage and electrochemistry applications. Chem. A 22, 153–159 (2016). https://doi.org/10.1002/chem.201503110
K. Dhara, T. Ramachandran, B.G. Nair, T.G. Satheesh Babu, Single step synthesis of Au–CuO nanoparticles decorated reduced graphene oxide for high performance disposable nonenzymatic glucose sensor. J. Electroanal. Chem. 743, 1–9 (2015). https://doi.org/10.1016/j.jelechem.2015.02.005
H. Yang, C. Shan, F. Li et al., Covalent functionalization of polydisperse chemically-converted graphene sheets with amine-terminated ionic liquid. Chem. Commun. (2009). https://doi.org/10.1039/b905085j
X. Bai, C. Sun, D. Liu et al., Photocatalytic degradation of deoxynivalenol using graphene/ZnO hybrids in aqueous suspension. Appl. Catal. B 204, 11–20 (2017). https://doi.org/10.1016/j.apcatb.2016.11.010
H. Sun, S. Liu, S. Liu, S. Wang, A comparative study of reduced graphene oxide modified TiO2, ZnO and Ta2O5 in visible light photocatalytic/photochemical oxidation of methylene blue. Appl. Catal. B 146, 162–168 (2014). https://doi.org/10.1016/j.apcatb.2013.03.027
M.E.D.G. Azenha, H.D. Burrows, L.M. Canle et al., Kinetic and mechanistic aspects of the direct photodegradation of atrazine, atraton, ametryn and 2-hydroxyatrazine by 254 nm light in aqueous solution. J. Phys. Org. Chem. 16, 498–503 (2003). https://doi.org/10.1002/poc.624
W. Wang, J. Yu, Q. Xiang, B. Cheng, Enhanced photocatalytic activity of hierarchical macro/mesoporous TiO2-graphene composites for photodegradation of acetone in air. Appl. Catal. B 119–120, 109–116 (2012). https://doi.org/10.1016/j.apcatb.2012.02.035
K. Zhou, Y. Zhu, X. Yang et al., Preparation of graphene–TiO2 composites with enhanced photocatalytic activity. N. J. Chem. 35, 353–359 (2011)
V.R. Posa, V. Annavaram, J.R. Koduru et al., Preparation of graphene–TiO2 nanocomposite and photocatalytic degradation of Rhodamine-B under solar light irradiation. J. Exp. Nanosci. 11, 722–736 (2016). https://doi.org/10.1080/17458080.2016.1144937
G. Peng, J.E. Ellis, G. Xu et al., In situ grown TiO2 nanospindles facilitate the formation of holey reduced graphene oxide by photodegradation. ACS Appl. Mater. Interfaces 8, 7403–7410 (2016). https://doi.org/10.1021/acsami.6b01188
G. Byzynski, C. Ribeiro, E. Longo, Blue to yellow photoluminescence emission and photocatalytic activity of nitrogen doping in TiO2 powders. Int. J. Photoenergy (2015). https://doi.org/10.1155/2015/831930
E. Cui, G. Lu, Enhanced surface electron transfer by fabricating a core/shell Ni@NiO cluster on TiO2 and its role on high efficient hydrogen generation under visible light irradiation. Int J Hydrog. Energy 39, 8959–8968 (2014). https://doi.org/10.1016/j.ijhydene.2014.03.258
D. Martín-Yerga, E.C. Rama, A. Costa-García, Electrochemical characterization of ordered mesoporous carbon screen-printed electrodes. J. Electrochem. Soc. 163, B176–B179 (2016). https://doi.org/10.1149/2.0871605jes
Soares GB, Ribeiro RAP, De Lazaro SR, Ribeiro C, Photoelectrochemical and theoretical investigation of the photocatalytic activity of TiO2: N. RSC Adv. (2016). https://doi.org/10.1039/c6ra15825k
J. Low, J. Yu, M. Jaroniec et al., Heterojunction photocatalysts. Adv. Mater. (2017). https://doi.org/10.1002/adma.201601694
G.B. Soares, R.A.P. Ribeiro, S.R. de Lazaro, C. Ribeiro, Photoelectrochemical and theoretical investigation of the photocatalytic activity of TiO2: N. RSC Adv. (2016). https://doi.org/10.1039/c6ra15825k
P. Wang, S. Zhan, Y. Xia et al., The fundamental role and mechanism of reduced graphene oxide in rGO/Pt–TiO2 nanocomposite for high-performance photocatalytic water splitting. Appl. Catal. B 207, 335–346 (2017). https://doi.org/10.1016/j.apcatb.2017.02.031
K.S. Divya, M.M. Xavier, P.V. Vandana et al., A quaternary TiO2/ZnO/RGO/Ag nanocomposite with enhanced visible light photocatalytic performance. N. J. Chem. 41, 6445–6454 (2017). https://doi.org/10.1039/C7NJ00495H
Y. Yang, Q. Jin, D. Mao et al., Dually ordered porous TiO2–rGO composites with controllable light absorption properties for efficient solar energy conversion. Adv. Mater. (2016). https://doi.org/10.1002/adma.201604795
X. Li, R. Shen, S. Ma et al., Graphene-based heterojunction photocatalysts. Appl. Surf. Sci. 430, 53–107 (2018). https://doi.org/10.1016/j.apsusc.2017.08.194
Acknowledgements
The authors acknowledge São Paulo Research Foundation (FAPESP), grant #CEPID 2013/07296-2, grant #2015/04511-5, grant #2014/17343-0, grant #2017/01267-1, grant #2012/26671-9. National Council for Scientific and Technological Development – CNPQ, grant #444926/2014-3. Authors thank Dr. Carlos J.L. Constantino and her student Sabrina A. Camacho for Raman measurements (FAPESP grant #2014/11410-8).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Byzynski, G., Volanti, D.P., Ribeiro, C. et al. Direct photo-oxidation and superoxide radical as major responsible for dye photodegradation mechanism promoted by TiO2–rGO heterostructure. J Mater Sci: Mater Electron 29, 17022–17037 (2018). https://doi.org/10.1007/s10854-018-9799-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-9799-0