Abstract
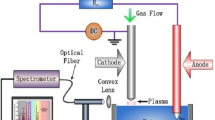
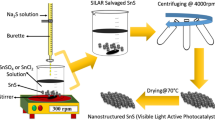
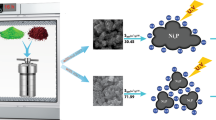
In current work, surfactant free synthesis of Sr2CeO4 nanostructures by co-precipitation process were reported. This method require lower firing temperature (700 °C) compare to the previous reports (above 1000 °C). This study shows that by controlling the reaction conditions such as kind of precipitating agent, surfactant and calcination temperature, the Sr2CeO4 nanostructures with various morphologies were prepared. Up to now, luminescence properties of Sr2CeO4 structures received great attention, while we focused on the photocatalytic activity of Sr2CeO4 nanostructures. Results show that the Sr2CeO4 nanostructures have exhibited excellent photodegradation performance of Acid red 88 azo dye as water pollutants under UV light irradiation.







Similar content being viewed by others
References
F. Ansari, F. Soofivand, M. Salavati-Niasari, Mater. Charact. 103, 11 (2015)
E. Danielson, M. Devenney, D.M. Giaquinta, J.H. Golden, R.C. Haushalter, E.W. Mc Farland, D.M. Poojary, C.M. Reaves, W.H. Weinberg, X.D. Wu, Science 279, 837 (1998)
L.V. Pieterson, S. Soverna, A. Meijering, J. Electrochem. Soc. 147, 4688 (2000)
Y.D. Jiang, F. Zhang, C.J. Summers, Z.L. Wang, Appl. Phys. Lett. 74, 1677 (1999)
J. Gomes, A.M. Pires, O.A. Serra, Quim. Nova. 27, 706 (2004)
C.H. Lu, C. Chen, J. Sol-Gel Sci. Technol. 43, 179 (2007)
N. Perea, G.A. Hirata, Thin Solid Films. 497, 177 (2006)
N. Perea, G.A. Hirata, Opt. Mater. 27, 1212 (2005)
Y. Tang, H. Guo, Q. Qin, Solid State Commun. 121, 351 (2002)
C.H. Park, C.H. Kim, C.H. Pyun, J.H. Choy, J. Lumin. 87–89, 1062 (2000)
Y.E. Lee, D.P. Norton, J.D. Budai, P.D. Rack, M.D. Potter, Appl. Phys. Lett. 77(5), 678 (2000)
O.A. Serra, V.P. Severino, P.S. Calefi, S.A. Cicillini, J. Alloys Compound. 323–324, 667 (2001)
X. Yu, X.H. He, S.P. Yang, X. Yang, X. Xu, Mater. Lett. 58, 48 (2003)
J. Gomes, A.M. Pires, O.A. Serra, Quim. Nova. 27, 5 (2004)
Y.B. Khollam, S.B. Deshpande, P.K. Khanna, P.A. Joy, H.S. Potdar, Mater. Lett. 58(20), 2521 (2004)
S.J. Chen, X.T. Chen, Z. Yu, J.M. Hong, Z. Xue, X.Z. You, Solid State Commun. 130(3–4), 48 (2004)
A. Nag, T.R.N. Kutty, J. Mater. Chem. 13, 370 (2003)
T. Hirai, Y. Kawamura, J. Phys. Chem. B. 108, 12763 (2004)
T. Hirai, Y. Kawamura, J. Phys. Chem. B. 109, 5569 (2005)
H. Kim, Y.T. Kim, H.K. Chae, Y. Dong, H. Yun, J. Sol–Gel Sci. Technol. 33, 75 (2005)
D.S. Xing, J.X. Shi, M.L. Gong, Mater. Lett. 59, 948 (2005)
F. Ansari, M. Bazarganipour, M. Salavati-Niasari, Mater. Sci. Semicond. Process. 43, 34 (2016)
F. Ansari, M. Salavati-Niasari, Adv. Powder Technol. 27, 2025 (2016)
M. Valian, F. Beshkar, M. Salavati-Niasari, J. Mater. Sci.: Mater. Electron. 28, 14996 (2017)
C.L. Marchena, L. Lerici, S. Renzini, L. Pierella, L. Pizzio, Appl. Catal. B: Environ. 188, 23 (2016)
S.A. Hassanzadeh-Tabrizi, M.M. Motlagh, S. Salahshour, Appl. Surf. Sci. 384, 237 (2016)
A. Mittal, J. Mittal, A. Malviya, V.K. Gupta, J. Colloid Interface Sci. 344, 497 (2010)
V.K. Gupta, S. Agarwal, T.A. Saleh, J. Hazard. Mater. 185, 17 (2011)
S. Zhang, X. Wang, J. Li, T. Wen, J. Xu, X. Wang, RSC Adv. 4, 63110 (2014)
S. Moshtaghi, S. Gholamrezaei, M. Salavati Niasari, P. Mehdizadeh, J. Mater. Sci.: Mater. Electron. 27, 414 (2016)
P.Z. Zambare, K.D. Girase, K.V.R. Murthy, O.H. Mahajan, Adv. Mater. Lett. 4(7), 577 (2013)
L.A. Rocha, M.A. Schiavon, C.S. Nascimento Jr, L. Guimarães, M.S. Góes, A.M. Pires, C.O. Paiva-Santos, O.A. Serra, M.A. Cebim, M.R. Davolos, J.L. Ferrari, J. Alloys Compd. 608, 73 (2014)
F. Namvar, F. Beshkar, M. Salavati-Niasari, S. Bagheri, J. Mater. Sci.: Mater. Electron. 28, 10313 (2017)
H. Najafian, F. Manteghi, F. Beshkar, M. Salavati-Niasari, Sep. Purif. Technol. 195, 30 (2018)
Acknowledgements
Authors are grateful to the council of Iran National Science Foundation (INSF) and University of Kashan for supporting this work by Grant No. (159271/822990).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Beshkar, F., Amiri, O., Salehi, Z. et al. Surfactant free synthesis of Sr2CeO4 nanostructures and their application in removal of organic pollutions. J Mater Sci: Mater Electron 29, 6978–6984 (2018). https://doi.org/10.1007/s10854-018-8684-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-8684-1