Abstract
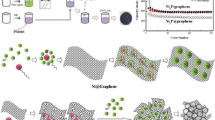
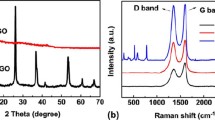
We reported a facile self-generated sacrificial template method for fabricating mesoporous three dimension NiCo2O4/graphene electrode material. Nickel, cobalt, and zinc ions dissolved in ethylene glycol reacted with potassium hydroxide solution to co-deposit onto graphene at 140 °C under atmospheric environment. With further addition of potassium hydroxide, zinc hydroxide as a self-generated sacrificial template was dissolved in situ, leading to the formation of mesoporous morphology. Structure and morphology characteristics were determined by X-ray diffraction, scanning electron microscopy, high-resolution transmission electron microscopy, and N2 adsorption experiments. Electrochemical properties were analyzed by AC impedance spectroscopy, cycling voltammetry, and charge/discharge test in 2 M KOH. Results showed that the as—prepared NiCo2O4/graphene electrode possessed a large specific surface area of 281.4 m2 g−1, an ultrahigh specific capacity of 1024.99 and 662.12 C g−1 at current density of 1 and 50 A g−1 respectively, and a long-term cycling life of 10,000 charge/discharge tests.










Similar content being viewed by others
References
R. Vellacheri, A. Al–Haddad, H. Zhao, W. Wang, C. Wang, Y. Lei, Nano Energy 8, 231 (2014)
J.R. Mille, P. Simon, Science 321, 651 (2008)
P.J. Hall, M. Mirzaeian, S.I. Fletcher, F.B. Sillars, A.J.R. Rennie, O. Shitta–Bey, G. Wilson, A. Cruden, R. Carter, Energy Environ. Sci. 9, 1238 (2012)
P. Chen, G. Shen, Y. Shi, H. Chen, C. Zhou, ACS Nano 4, 4403 (2012)
K. Liang, L. Lei, Y. Yang, ACS Energy Lett. 2, 373 (2017)
H. Xia, Y. Meng, G. Yua, C. Cui, L. Lu, J. Solid State Electrochem. 15, A60 (2012)
N. Wang, P. Zhao, K. Liang, M. Yao, Y. Yang, W. Hu, Chem. Eng. J. 207, 105 (2017)
T. Brousse, D. Belanger, J. Long, J. Electrochem. Soc. 162, A5185 (2015)
P. Simon, Y. Gogotsi, B. Dunn, Science 343, 1210 (2014)
B. Saravanakumar, K.K. Purushothaman, G. Muralidharan, ACS Appl. Mater. Interfaces 4, 4484 (2012)
M. Zhou, Y. Deng, X. Liu, W. Hu, J. Mater. Sci.: Mater. Electron. 26, 6306 (2015)
K. Liang, X. Tang, W. Hu, J. Mater. Chem. 22, 11062 (2012)
M. Zhou, Y. Deng, K. Liang, X. Liu, B. Wei, W. Hu, J. Electroanal. Chem. 742, 1 (2015)
X. Xia, J. Tu, Y. Zhang, X. Wang, C. Gu, X. Zhao, H. Fan, ACS Nano 6, 5531 (2012)
Y. Yuan, X. Xia, J. Wu, X. Huang, Y. Pei, J. Yang, S. Guo, Electrochem. Commun. 13, 1123 (2011)
L. Li, P. Gao, S. Gai, F. He, Y. Chen, M. Zhang, P. Yang, Electrochim. Acta 190, 566 (2016)
L. Chang, F. Ren, C. Zhao, X. Xue, J. Electroanal. Chem. 778, 110 (2016)
H. Wang, H. Casalongue, Y. Liang, H. Dai, J. Am. Chem. Soc. 132, 7472 (2012)
L. Cao, F. Xu, Y. Liang, H. Li, Adv. Mater. 16, 1853 (2014)
T. Xue, X. Wang, J. Lee, J. Power Sources 201, 382 (2012)
K. Adib, M. Rahimi-Nasrabadi, Z. Rezvani, S.M. Pourmortazavi, F. Ahmadi, H.R. Naderi, M.R. Ganjali, J. Mater. Sci.: Mater. Electron. 27, 4541 (2016)
J. Yin, H. Zhang, J. Luo, M. Yao, W. Hu, J. Mater. Sci.: Mater. Electron. 28, 2093 (2017)
L. Hu, L. Wu, M. Liao, X. Hu, X. Fang, Adv. Funct. Mater. 22, 998 (2012)
X. Wang, X. Han, M. Lim, N. Singh, C. Gan, M. Jan, P.S. Lee. J. Phys. Chem. C. 116, 12448 (2012)
D.U. Lee, B.J. Kim, Z. Chen, J. Mater. Chem. A. 1, 4754 (2013)
N. Wang, P. Zhao, Q. Zhang, M. Yao, W. Hu, Compos. Part B-Eng. 113, 144 (2017)
E. Jokar, A.I. Zad, S. Shahrokhian J. Solid State Electrochem. 19, 269 (2015)
R.B. Waghmode, A.P. Torane, J. Mater. Sci.: Mater. Electron. 27, 6133 (2016)
X. Wang, C. Yan, A. Sumboja, P. Lee, Nano Energy 3, 119–126 (2014)
Y. Bai, B. Wang, X. Lun, J. Sun, L. Gao, J. Colloid Interf. Sci. 468, 1 (2016)
Q. Zhang, N. Wang, P. Zhao, M. Yao, W. Hu, Compos. Part A. 98, 58, (2017)
H. Zhou, H. Zhai, G. Han, J. Mater. Sci.: Mater. Electron. 27, 2773 (2016)
C. Lee, X. Wei, J.W. Kysar, J. Hone, Science 321, 385 (2008)
H. Jiang, P.S. Lee, C. Li, Energy Environ. Sci. 6, 41 (2013)
S. Ye, L.G. Kim, S.Y. Yang, J.W. Lee, W.C. Oh, J. Mater. Sci.: Mater. Electron. (2017). doi:10.1007/s10854-017-6349-0
B. Wei, L. Wang, Q. Miao, Y. Yuan, P. Dong, R. Vajtai, W.D. Feia, Carbon 85, 249 (2015)
V.H. Nguyen, C. Lamiel, J. Shim, Mater. Lett. 170, 105 (2016)
S. Beraa, H. Khana, I. Biswas, S. Jana Appl. Surf. Sci. 383, 165 (2016)
J. Rouquerol, D. Avnir, C.W. Fairbridge, D.H. Everett, J.M. Haynes, N. Pernicone, J.D.F. Ramsay, K.S. Sing, W.K.K. Unger, Pure. Appl. Chem. 66, 1739 (1994)
V. Gupta, S. Gupta, N. Miura, J. Power Sources 175, 680 (2008)
C. Hu, C. Cheng, Electrochem. Solid–State Lett. 5, A43 (2002)
M. Yao, N. Wang, W. Hu, J. Electroanal. Chem. 782, 133 (2016)
Y. Shang, Y. Gai, L. Wang, L. Hao, H. Lv, F. Dong, L. Gong, Eur. J. Inorg. Chem. doi:10.1002/ejic.201700020 (2017)
N. Wang, M. Yao, P. Zhao, W. Hu, S. Komarneni, J. Mater. Chem. A. 5, 5838 (2017)
D. Zhao, J. Qin, L. Zheng, M. Cao, Chem. Mater. 28, 4180 (2016)
Acknowledgements
We gratefully acknowledge the funding support by Laboratory of Precision Manufacturing Technology, CAEP (Grant No. KF15003).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yao, M., Wang, N., Yin, J. et al. Mesoporous three dimension NiCo2O4/graphene composites fabricated by self-generated sacrificial template method for a greatly enhanced specific capacity. J Mater Sci: Mater Electron 28, 11119–11124 (2017). https://doi.org/10.1007/s10854-017-6898-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-6898-2