Abstract
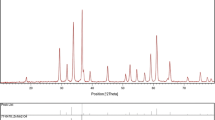
Nowadays, lead oxide is one of the most important materials that have a lot of electrochemical and industrial applications such as oxidation of organic compounds in waste water, oxidation of phenol, Cr3+, and glucose, evolution of ozone, and as an electrocatalyst for salicylic acid. In this work, lead oxide nanocrystals were prepared by hydrothermal method in mild condition and the effect of various kinds of capping agent was investigated to achieve lower than 10 nm nanoparticle. Photocatalytic property of these nanoparticles in different dyes degradation under UV light was investigated. Nanoparticles were characterized by using X-ray diffraction, scanning electron microscopy, transmission electron microscopy, Fourier transform infrared techniques X-ray energy dispersive spectroscopy and Ultraviolet spectroscopy (UV–Vis).






Similar content being viewed by others
References
K. Kannan, G. Muthuraman, S. Moon, Controlled synthesis of highly spherical nano-PbO2 particles and their characterization. Mater. Lett. 123, 19–22 (2014)
X. Yang, R. Zou, F. Huo, D. Cai, D. Xiao, Preparation and characterization of Ti/SnO2–Sb2O3–Nb2O5/PbO2 thin film as electrode material for the degradation of phenol. J. Hazard. Mater. 164, 367–373 (2009)
D. Devilliers, M. Dinh Thi, E. Mahe, Q. Le Xuan, Cr(III) oxidation with lead dioxide-based anodes. Electrochim. Acta 48, 4301 (2003)
T.V. Kasumov, Synthesis, spectroscopic characterization and ESR studies on electron transfer reactions of bis[N-(2,6-di-tert-butyl-1-hydroxyphenyl)salicylaldiminato]-copper(II) complexes with PbO2 and PPh3. Spectrochimica Acta Part A 57, 1649–1662 (2001)
P. Gao, Y. Liu, X. Bu, M. Hu, Y. Dai, X. Gao, L. Lei, A non-conventional fluorinated separator in high-voltage graphite/LiNi0.4Mn1.6O4 cells. J. Power Source 242, 299–304 (2013)
A. Al-Saie, A. Jaafar, M. Bououdina, Structure and magnetic properties of mechanically milled Pb3O4–Fe2O3 mixture. Int. J. Nanoparticles 4, 20–26 (2011)
L.X. Ding, F.L. Zheng, J.W. Wang, G.R. Li, Z.L. Wang, Y.X. Tong, Selected-contr Super-large dendrites composed of trigonal PbO2 nanoplates with enhanced performances for electrochemical devices, synthesis of PbO2 submicrometer-sized hollow spheres and Pb3O4 microtubes. Chem. Commun. 48, 1275–1277 (2012)
G. Xi, Y. Peng, L. Xu, M. Zhang, W. Yu, Y. Qia, Selected-control synthesis of PbO2 submicrometer-sized hollow spheres and Pb3O4 microtubes. Inorg. Chem. Commun. 7, 607–610 (2004)
M. Bervas, M. Perrin, S. Genies, F. Mattera, Low-cost synthesis and utilization in mini-tubular electrodes of nano PbO2. J. Power Source 173, 570–577 (2007)
T.I. Dyuzheva, L.M. Lityagina, N.A. Bendeliani, Hydrothermal crystal growth of the high-pressure phases of α-PbO2 and TiO2. J. Alloy Compd. 377, 17–20 (2004)
N. Fan, C. Sun, D. Kong, Y. Qian, Chemical synthesis of PbO2 particles with multiple morphologies and phases and their electrochemical performance as the positive active material. J. Power Source 254, 323–328 (2014)
S. Ghasemi, M.F. Mousavi, M. Shamsipur, H. Karami, Sonochemical-assisted synthesis of nano-structured lead dioxide. Ultrason. Sonochem. 15, 448–455 (2008)
F.K. Butt, C. Cao, W.S. Khan, Z. Ali, T. Mahmood, R. Ahmed, S. Hussain, G. Nabi, Fabrication of novel SnO2 nanofibers bundle and their optical properties. Mater. Chem. Phys. 136, 10–14 (2012)
G. Xi, Y. Peng, Li.. Xu, M. Zhang, W. Yu, Y. Qian, Selected-control synthesis of PbO2 submicrometer-sized hollow spheres and Pb3O4 microtubes. Inorg. Chem. Commun. 7, 607–610 (2004)
M. Salavati-Niasari, B. Shoshtari-Yeganeh, F. Mohandes, Schiff-base assisted synthesis of lead selenide nanostructures. Mater. Res. Bull. 48, 1745–1752 (2013)
S. Gholamrezaei, M. Salavati-Niasari, M. Bazarganipour, M. Panahi-Kalamaoui S. Bagheri, Novel precursors for synthesis of dendrite-like PbTe nanostructures and investigation of photoluminescence behavior. Adv. Powder. Technol. 25, 1585–1592 (2014)
S. Gholamrezaei, M. Salavati-Niasari, D. Ghanbari, A facile hydrothermal method for synthesis different morphologies of PbTe nanostructures. J. Ind. Eng. Chem. 20, 3335–3351 (2014)
S. Gholamrezaei, M. Salavati-Niasari, D. Ghanbari, Synthesis and application of lead telluride nanoparticles for degradation of organic pollution. J. Ind. Eng. Chem. 20, 4000–4007 (2014)
B. Han, W. Zhao, X. Qin, Y.G. Li, Y. Sun, W. Wei, Synthesis of dimethyl hexane-1,6-diyldicarbamate from 1,6-hexamethylenediamine and methyl carbamate using lead dioxide as catalyst. Catal. Commun 33, 38–41 (2013)
X. Duan, F. Mab, Z. Yuan, L. Chang, X. Jin, Electrochemical degradation of phenol in aqueous solution using PbO2 anode. J. Taiwan Inst. Chem. Eng. 44, 95–102 (2013)
L. Nejati-Moghadam, D. Ghanbari, M. Salavati-Niasari, A. Esmaeili-Bafghi-Karimabad, S. Gholamrezaei, Photo-degradation of organic dyes: simple chemical synthesis of various morphologies of tin dioxide semiconductor and its nanocomposite, J. Mater. Sci. 26, 6386–6394 (2015)
M. Goudarzi, D. Ghanbari, M. Salavati-Niasari, Room temperature preparation of aluminum hydroxide nanoparticles and flame retardant poly vinyl alcohol nanocomposite. J. Nanostruct. 5, 105–110 (2015)
A. Esmaeili-Bafghi-Karimabad, D. Ghanbari, M. Salavati-Niasari, H. Safardoust-Hojaghan, Microwave-assisted synthesis of SiO2 nanoparticles and its application on the flame retardancy of poly styrene and poly carbonate nanocomposites. J. Nanostruct. 5, 263–269 (2015)
S. Moshtaghi, M. Salavati-Niasari, D. Ghanbari, Characterization of CaSn(OH)6 and CaSnO3 nanostructures synthesized by a new precursor. J. Nanostruct. 5, 169–174 (2015)
F. Beshkar, M. Salavati-Niasari, Facile synthesis of Nickel Chromite nanostructures by hydrothermal route for photocatalytic degradation of acid black 1 under visible light. J. Nanostruct. 5, 17–23 (2015)
M. Mousavi-Kamazani, M. Salavati-Niasari, D. Ghanbari, A facile solvothermal method for synthesis of CuInS2 nanostructures. J. Nanostruct. 2, 363–368 (2012)
Acknowledgements
Authors are grateful to the council of Iran National Science Foundation (INSF) and University of Kashan for supporting this work by Grant No. (159271/529).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nejati-Moghadam, L., Gholamrezaei, S., Salavati-Niasari, M. et al. Hydrothermal synthesis and characterization of lead oxide nanocrystal in presence of tetradentate Schiff-base and degradation investigation of organic pollutant in waste water. J Mater Sci: Mater Electron 28, 9919–9926 (2017). https://doi.org/10.1007/s10854-017-6748-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-6748-2