Abstract
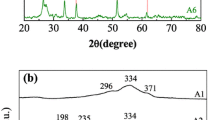
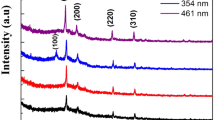
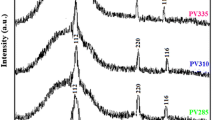
Stannite and wurtzite Cu2FeSnS4 (CFTS) thin films were synthesized directly on glass substrates via the solvothermal method firstly. The solvent plays an important role in the formation and morphologies of two different CFTS phases. X-ray diffraction, Raman spectroscopy, scanning electron microscopy, UV–Vis–NIR absorbance spectroscopy measurement and Hall effect measurement show that the surface of stannite CFTS thin film is covered with large numbers of irregular particles while wurtzite CFTS thin film exhibits sphere-like particles. The stannite and wurtzite CFTS thin films show that the carrier concerntration is in the range of 1018cm−3 and carrier mobility of about 4.602–21.98 cm−2 v−1 s−1. The band gaps of stannite and wurtzite CFTS thin films are determined to 1.3 and 1.34 eV, repectively, which are suitable as a substitute for thin film solar cells.




Similar content being viewed by others
References
P. Jackson, D. Hariskos, E. Lotter, S. Paetel, R. Wuerz, R. Menner, W. Wischmann, M. Powalla, Prog. Photovolt. 19, 894–897 (2011)
B. Shin, O. Gunawan, Y. Zhu, N.A. Bojarczuk, S.J. Chey, S. Guha, Prog. Photovolt. 21, 72–76 (2013)
T.K. Todorov, J. Tang, S. Bag, O. Gunawan, T. Gokmen, Y. Zhu, D.B. Mitzi, Adv. Energy. Mater. 3, 34–38 (2013)
X.Y. Zhang, N.Z. Bao, K. Ramasamy, Y.H.A. Wang, Y.F. Wang, B.P. Lin, A. Gupta, Chem. Commun. 48, 4956–4958 (2012)
L. Li, X.Y. Liu, J. Huang, M. Cao, S.Y. Chen, Y. Shen, L.J. Wang, Mater. Chem. Phys. 133, 688–691 (2012)
C. Yan, C. Huang, J. Yang, F.Y. Liu, J. Liu, Y.Q. Lai, J. Li, Y.X. Liu, Chem. Commun. 48, 2603–2605 (2012)
X. Jiang, W. Xu, R.Q. Tan, W.J.M. Song, J. Chen, Mater. Lett. 102–103, 39–42 (2013)
J.S. Zhong, Q.Y. Wang, D.Q. Chen, L.F. Chen, H. Yu, H.W. Lu, Z.G. Ji, Appl. Surf. Sci. 343, 28–32 (2015)
M. Cao, C. Li, B.L. Zhang, J. Huang, L.J. Wang, Y. Shen, J. Alloys Compd. 622, 695–702 (2015)
L.H. Ai, J. Jiang, Nanotechnology 23, 495601–495609 (2012)
W. Wang, H.L. Shen, H.Y. Yao, J.Z. Li, Mater. Lett 125, 183–186 (2014)
Z. Gui, R. Fan, X.H. Chen, Y. Hu, Z.Z. Wang, Mater. Res. Bull. 39, 237–241 (2004)
X. Fontané, V. Izquierdo-Roca, E. Saucedo, S. Schorr, V.O. Yukhymchuk, M.Y. Valakh, J.R. Morante, J. Alloys Compd. 539, 190–194 (2012)
H. Guan, H.L. Shen, B.X Jiao, X. Wang, Mater. Sci. Semicond. Process 25, 159–162 (2014)
D.B. Khadka, J.H. Kim, J. Phys. Chem. C 118, 14227–14237 (2014)
X. Fontane, V. Izquierdo-Roca, E. Saucedo, S. Schorr, V.O. Yukhymchuk, M.Y. Valakh, A. Perez-Rodriguez, J.R. Morante, J. Alloys Compd. 539, 190–194 (2012)
D.B. Khadka, J. Kim, J. Alloys Compd. 638, 103–108 (2015)
S.Y. Chen, A. Walsh, Y. Luo, J.H. Yang, X.G. Gong, S.H. Wei, Phys. Rev. B 82, 195203 (2010)
Acknowledgements
This research is financial supported by the National Natural Science Foundation of China (No.51402251).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hou, H., Guan, H. & Li, L. Synthesis of Cu2FeSnS4 thin films with stannite and wurtzite structure directly on glass substrates via the solvothermal method. J Mater Sci: Mater Electron 28, 7745–7748 (2017). https://doi.org/10.1007/s10854-017-6469-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-6469-6