Abstract
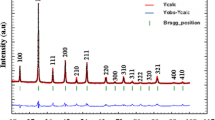
The lead-free Ba(Ti0.96Mg0.013Nb0.026)O3 composition has been prepared by solid state reaction. The room temperature X-ray diffraction revealed a perovskite phase with a tetragonal symmetry. The complex dielectric permittivity measured on cooling from 470 to 150 K in the frequency range (102–106 Hz) indicated a ferroelectric behavior and exhibited a large electromechanical response. This ferroelectric perovskite showed photoelectrochemical properties with an optical gap of 2.90 eV, n-type conduction and a flat band potential of −0.57 V SCE . As application, the oxide is successfully tested for the eosin oxidation under solar light. At pH ~ 6.3, 90 % of eosin (15 mg L−1) disappeared after 6 h of illumination for a catalyst dose of 2.5 g L−1.










Similar content being viewed by others
References
J.L. Giocondi, G.S. Rohrer, Spatial separation of photochemical oxidation and reduction reactions on the surface of ferroelectric BaTiO3. J. Phys. Chem. B 105, 8275 (2001)
N.V. Burbure, P.A. Saladore, G.S. Rohrer, Photochemical reactivity of titania films on BaTiO3 substrates: origin of spatial selectivity. Chem. Mater. 22, 5823 (2010)
K. Lin, J. Xing, W. Wang, Z. Shan, F. Xu, F. Huang, Photocatalytic activities of heterojunction Bi2O3/BaTiO3: a strategy for the design of efficient combined catalyst. J. Phys. Chem. C 111, 18288 (2007)
G.A. Smolenskii, A.I. Agranovskaya, Dielectric polarization of a number of complex compounds. Sov. Phys. Solid State 1, 1429 (1959)
L.E. Cross, Relaxor Ferroelectrics. Ferroelectrics 76, 241 (1987)
Y. Yang, Y. Sun, Y. Jiang, Structure and photocatalytic property of perovskite and perovskites-related compounds. Mater. Chem. Phys. 96, 234 (2006)
Z.H. Li, Y.X. Wang, J.W. Liu, G. Chen, Y. Li, C. Zhou, Photocatalytic hydrogen production from aqueous methanol solutions under visible light over Na(Bi x Ta1−x )O3 solid-solution. Int. J. Hydrog. Energy 34, 147 (2009)
A. Kerfah, K. Taïbi, S. Omeiri, M. Trari, Relaxor ferroelectric and photocatalytic behaviour of Ba0.785Bi0.127Y0.017 TiO3 composition. Sol. Energy 85, 443 (2011)
N. Boutal, G. Rekhila, K. Taïbi, M. Trari, Relaxor ferroelectric and photoelectrochemical properties of lead-free Ba1−xEu(Ti0.75Zr0.25)O3 ceramics. Application to chromate reduction. Sol. Energy 99, 291 (2014)
B. Jaffe, W.R. Cook, H. Jaffe, Piezoelectric Ceramics (Academic, London, 1971), p. 317
J.H. Paik, S. Nahm, J.D. Bylin, M.H. Kim, H.J. Lee, The effect of Mg deficiency on the microwave dielectric properties of BaMg1/3Nb2/3O3. J. Mater. Sci. Lett. 17, 1777 (1998)
X. Chou, J. Zhai, H. Jiang, X. Yao, Dielectric properties and relaxor behaviour of rare-earth (La, Sm, Eu, Dy, Y) substituted barium zirconium titanate ceramics. Appl. Phys. 102, 84106 (2007)
A. Simon, J. Ravez, M. Maglione, Relaxor properties of Ba1−x Bi0.067(Ti1−xZrx)O3 ceramics. Solid State Sci. 7, 925 (2005)
J. Ravez, A. Simon, Some solid state chemistry aspect. J. Solid State Chem. 162, 260 (2001)
C.A. Randall, A.S. Bahlla, Nanostructural-property relations in complex lead perovskites. Jpn. J. Appl. Phys. 29, 327 (1990)
M. Goudarzi, M. Salavati-Niasari, S.M. Hosseinpour-Mashkani, N. Mir, Controlled synthesis of Tl2O3 nanostructures via microwave route by a novel pH adjuster and investigation of its photocatalytic activity. J. Mater. Sci. Mater. Electron. 26, 5326 (2015)
M. Ramezani, A. Davoodi, A. Malekizad, S.M. Hosseinpour-Mashkani, Synthesis and characterization of Fe2TiO5 nanoparticles through a sol–gel method and its photocatalyst applications. J. Mater. Sci. Mater. Electron. 26, 3957 (2015)
M. Ramezani, A. Sobhani-Nasab, S.M. Hosseinpour-Mashkani, Synthesis, characterization and morphological control of Na1/2Bi1/2Cu3Ti4O12 through modify sol–gel method. J. Mater. Sci. Mater. Electron. 26, 4848 (2015)
M. Ramezani, S.M. Hosseinpour-Mashkani, A. Sobhani-Nasab, H.G. Estarki, Synthesis, characterization, and morphological control of ZnMoO4 nanostructures through precipitation method and its photocatalyst application. J. Mater. Sci. Mater. Electron. 26, 7588 (2015)
M. Behpour, M. Mehrzad, S.M. Hosseinpour-Mashkani, TiO2 thin film: preparation, characterization, and its photocatalytic degradation of basic yellow 28 dye. J.N.S. 5, 183 (2015)
H. Mekatel, S. Amokrane, B. Bellal, M. Trari, D. Nibou, Chem. Eng. J. 15, 611 (2012)
A. Boultif, D. Louer, J. Appl, Powder pattern indexing with the dichotomy method. J. Appl. Crystallogr. 37, 724 (2004)
G.H. Kwei, A.C. Lawson, S.J.L. Billinge, Structures of the ferroelectric phases of barium titanate. J. Phys. Chem. 97, 2368 (1993)
R.D. Shannon, C.T. Prewitt, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751 (1976)
A. Verbaere, Y. Piffard, Z.G. Ye, E. Housson, Lead magnoniobiate crystal structure determination. Mat. Res. Bull. 27, 1227 (1992)
A.K. Singh, D. Pandey, Evidence for MB and MC phases in the morphotropic phase boundary region of (1 − x)[Pb (Mg1/3Nb2/3)O3] − x PbTiO3: a Rietveld study. Phys. Rev. B 67, 064102 (2003)
G. Xu, G. Shirane, J.R.D. Copley, P.M. Gehring, Neutron elastic diffuse scattering study of Pb (Mg1/3Nb2/3) O3. Phys. Rev. B 69, 064112 (2004)
S.C. Abrahams, S.K. Kurtz, P.B. Jamieson, Atomic displacement relationship to Curie temperature and spontaneous polarization in displacive ferroelectrics. Phys. Rev. 172, 551 (1968)
W.M. Haynes (ed.), Handbook of Chemistry and Physics (CRC Press, Gladstone, 2014–2015)
A.H. Ghanbari Niaki, A.M. Bakhshayesh, M.R. Mohammad, Sol. Energy 103, 210 (2014)
Y.-D. Zhang, X.-M. Huang, D.-M. Li, Y.-H. Luo, Q.-B. Meng, Sol. Energy Mater. Sol. Cells 98, 417 (2012)
D. Nibou, H. Mekatel, S. Amokrane, M. Barkat, M. Trari, Adsorption of Zn2+ ions onto NaA and NaX zeolites: kinetic, equilibrium and thermodynamic studies. J. Hazard. Mater. 173, 637 (2010)
B. Hadjarab, M. Trari, M. Kebir, Mater. Sci. Semicond. Process. 29, 283 (2015)
Acknowledgments
The authors are grateful to Dr. B. Bellal for his valuable assistance in optical measurements. This work was supported financially by the Faculty of Chemistry (USTHB, Algiers). The authors wish to express their sincere thanks to the Electronic Ceramics Department of Jozef Stefan Institute (Ljubjana, Slovenia) for the use of their facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bensemma, N., Rekhila, G., Boutal, N. et al. Photoelectrochemical properties of lead-free ferroelectric ceramic Ba(Ti0.96Mg0.013Nb0.026)O3: application to solar conversion of eosin. J Mater Sci: Mater Electron 27, 6757–6765 (2016). https://doi.org/10.1007/s10854-016-4625-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-4625-z