Abstract
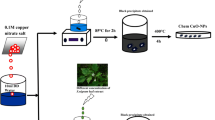
Cu nanoparticles (CuNPs) were loaded on the surface of hollow glass microspheres (HGMs) by electroless plating technique. The surface morphology and composition of the plated HGMs were characterized by XRD, XPS and SEM. The results show that the size and distribution of the CuNPs varied with the different copper sulfate concentrations and played an important part in photocatalytic hydrogen production. When the concentration was 2 g/L, the hydrogen production reached 3845 μmol/g h with lactic acid as a sacrificial agent and exhibited great stability for at least 20 h, which is better than the freshly synthesized alone CuNPs (1500 μmol/g h). What’s more, the photocatalytic degradation rate of methyl orange reached up to 95.75 % after 2 h under light irradiation and the photocatalysts can be recycled for further use.









Similar content being viewed by others
References
G. Tobias, M. Mariem, T. Harun (2015). doi:10.1002/asia.201500723
B. Liu, Y.L. Fang, Z.Y. Li, S. Xu, J. Nanosci. Nanotechnol. 15, 889 (2015)
M. Yoon, J.E. Lee, Y.J. Jang, J.W. Lim, A. Rani, D.H. Kim, A.C.S. Appl, ACS Appl. Mater. Interfaces 7, 21073 (2015)
Z. Chehadi, N. Alkees, A. Bruyant, J. Toufaily, J.S. Girardon, M. Capron, F. Dumeignil, T. Hamieh, R. Bachelot, S. Jradi, Mat. Sci. Semicond. Process. (2015). doi:10.1016/j.mssp.2015.08.044
S. Vadivel, G. Rajarajan, J. Mater. Sci.: Mater. Electron. 26, 5863 (2015)
J.M. Valero, O. Sergio, C. Gerardo, ACS Catal. 4, 3320 (2014)
G. Paramasivan, H. Katsumasa, K. Hideyuki, S. Tohru, F. Kunihiro, K. Satoshi, y 38, 11840 (2013)
H.Y. Liu, T.T. Wang, H.P. Zeng, Part. Part. Syst. Charact. 32, 869 (2015)
Q.Y. Zhang, M. Wu, W. Zhao, Surf. Coat. Technol. 192, 213 (2005)
X.B. Qi, C. Gao, Z.W. Zhang, S.F. Chen, B. Li, S. Wei, Int. J. Hydrogen Energy 37, 1518 (2012)
P. Shrivastava, S. Dalai, P. Sudera, S. Vijayalakshmi, P. Sharm, Microelectron. Eng. 126, 103 (2014)
X.J. Duan, R.J. Gao, Y.D. Zhang, Z. Jian, Mater. Lett. 65, 3625 (2011)
J.P. Huo, L.T. Fang, Y.L. Lei, G.C. Zeng, H.P. Zeng, J. Mater. Chem. A. 2, 11040 (2014)
J.P. Huo, H.P. Zeng, J. Mater. Chem. A. 3, 6258 (2015)
S. Shukla, S. Seal, Z. Rahaman, K. Scammon, Mater. Lett. 57, 151 (2002)
X.G. Cao, H.Y. Zhang, Patent No. CN1974460A
S.L. Xu, X. Sun, H. Ye, T. You, X.Y. Song, S.X. Sun, Mater. Chem. Phys. 120, 1 (2010)
P.D. Kirsch, J.G. Ekerdt, J. Appl. Phys. 90, 4256 (2001)
T. Ghodselahi, Appl. Surf. Sci. 255, 2730 (2008)
X.Z. Li, F.B. Li, C.L. Yang, W.K. Ge, J. Photoch, J Photochem. Photobiol. A. 141, 209 (2001)
D.Z. Li, W.Z. Wang, D. Jiang, Y.L. Zheng, X.M. Li, RSC Adv. 5, 14374 (2015)
M. Zhang, D.D. Lu, Z.H. Zhang, J.J. Yang, J. Electrochem. Soc. 162, 557 (2015)
W. Cheng, T. Yu, K. Chao, S. Lu, Chem. Cat. Chem. 6, 293 (2014)
J.M. Kum, Y.J. Park, H.J. Kim, S.O. Cho, Nanotechnology 26, 125402 (2015)
G.H. Chan, J. Zhao, E.M. Hicks, G.C. Schatz, R.P. Van Duyne, Nano Lett. 7, 1947 (2007)
E.Z. Liu, X.Y. Hu, Y. Hu, H. Li, C.N. Tang, L. Sun, J. Wan, J. Mater. Sci. 50, 2298 (2015)
Acknowledgments
The authors acknowledge financial support from the National Natural Science Foundation of China (Nos. 21371060, 21571064), the Pearl River S&T Nova Program of Guangzhou (No. 2014J2200047) and the Fundamental Research Funds for the Central Universities (No. 2015ZM162).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhao, K., Liu, H., Wang, T. et al. Cu-plated hollow glass microspheres for hydrogen production and degradation. J Mater Sci: Mater Electron 27, 5183–5189 (2016). https://doi.org/10.1007/s10854-016-4411-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-4411-y