Abstract
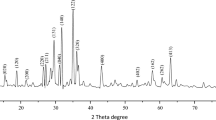
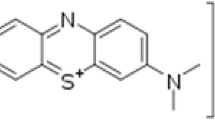
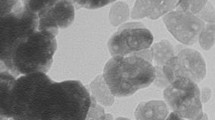
In this study, ZnO nanoparticles were fabricated by co-precipitation method. The synthesized nanoparticles possessed monodispersity with the average size 20–30 nm. Since the industrial effluents may not be at neutral pH, the effect of pH on the rate of degradation is important and need to be considered. In order to investigate the effect of pH on ZnO nanoparticles photocatalytic activity, the photocatalytic degradation of Rose Bengal, Methylene blue, and Bromocresol green dyes, was studied with different pH values. It was observed that the adsorption of the dyes onto ZnO nanoparticles surface is strongly dependent on the pH of the solution which plays an important role in photocatalytic degradation.













Similar content being viewed by others
References
Q.I. Rahman, M. Ahmad, S.K. Misra, M. Lohani, Mater. Lett. 91, 170 (2013)
S.P. Buthelezi, A.O. Olaniran, B. Pillay, Molecules 17, 14260 (2012)
A.M. Abdulkarem, E.M. Elssfah, N.-N. Yan, G. Demissie, Y. Yu, J. Phys. Chem. Solids 74, 647 (2013)
S. Suwanboon, P. Amornpitoksuk, A. Sukolrat, N. Muensit, Ceram. Int. 39, 2811 (2013)
X. Cai, Y. Cai, Y. Liu, H. Li, F. Zhang, Y. Wang, J. Phys. Chem. Solids 74, 1196 (2013)
T. Madrakian, A. Afkhami, M. Ahmadi, Spectrochim. Acta A 99, 102 (2012)
I.A. Siddiquey, T. Furusawa, M. Sato, N.M. Bahadur, M.M. Alam, N. Suzuki, Ultrason. Sonochem. 19, 750 (2012)
G.M. Nair, M. Nirmala, K. Rekha, A. Anukaliani, Mater. Lett. 65, 1797 (2011)
R. Yousefi, F. Jamali-Sheini, M. Cheraghizade, S. Khosravi-Gandomani, A. Sáaedi, N.M. Huang, W.J. Basirun, M. Azarang, Mater. Sci. Semicond. Process. 32, 152 (2015)
M. Azarang, A. Shuhaimi, R. Yousefi, A.M. Golsheikh, M. Sookhakian, Ceram. Int. 40, 10217 (2014)
F. Feng, C. Hao, H. Zhang, W. Xie, X. Wang, Y. Zhao, J. Mater. Sci.: Mater. Electron. 26, 6704 (2015)
S.V. Elangovan, N. Sivakumar, V. Chandramohan, J. Mater. Sci.: Mater. Electron. 26, 8753 (2015)
I. Kazeminezhad, A. Sadollahkhani, M. Farbod, Mater. Lett. 92, 29 (2013)
K. Vignesh, A. Suganthi, M. Rajarajan, S.A. Sara, Powder Technol. 224, 331 (2012)
J. Tauc, A. Menth, J. Non-Cryst. Solids 8, 569 (1972)
S.M. Lam, J.C. Sin, A.Z. Abdullah, A.R. Mohamed, Desalination 41, 131 (2012)
U.G. Akpan, B.H. Hameed, Hazard. Mater. 170, 520 (2009)
L.G. Devi, K.M. Reddy, Appl. Surf. Sci. 256, 3116 (2010)
X. Li, Y. Hou, Q. Zhao, L. Wang, J. Colloid Interface Sci. 358, 102 (2011)
Acknowledgments
The authors would like to thank Shahid Chamran University of Ahvaz for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kazeminezhad, I., Sadollahkhani, A. Influence of pH on the photocatalytic activity of ZnO nanoparticles. J Mater Sci: Mater Electron 27, 4206–4215 (2016). https://doi.org/10.1007/s10854-016-4284-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-4284-0