Abstract
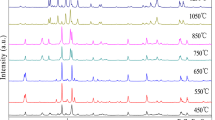
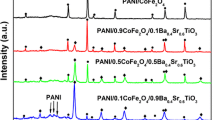
BaTiO3/BaZn2Fe16O27 composites were prepared by traditional solid state method, BaTiO3 nano particles were coated around with the BaZn2Fe16O27 ferrite with hexagonal plate structure. The prepared composite particles were characterized with X-ray diffraction, scanning electron microscopy, and vector network analyzer. With the increase of BaTiO3 content, the complex permittivity of the composites increases, while the complex permeability decreases; The sharp peaks of the magnetic loss appear at 8.72 and 14.0 GHz, and the peak values are 1.64 and 0.69, respectively, when the mole ratio of BaZn2Fe16O27/BaTiO3 is 10:5; The matching thickness of BaTiO3/BaZn2Fe16O27 composites is 2.5 mm when the mole ratio of BaZn2Fe16O27/BaTiO3 is 10:5, and the minimum reflection loss reaches −33.49 dB at 8.56 GHz; There are more than one loss peak appeared in the reflection loss cures, and the effective absorption frequency (RL < −10 dB) ranges from 7.92 to 14.96 GHz with a thickness of 3 mm.







Similar content being viewed by others
References
A.L. Xia, S.K. Liu, C.G. Jin, S.B. Su, J. Mater. Sci. Mater. Electron. 23, 4166 (2013). doi:10.1007/s10854-013-1377-x
A. Mukhtar, R. Grössinger, M. Kriegisch, F. Kubel, M.U. Rana, Curr. Appl. Phys. 12, 1413 (2012). doi:10.1016/j.cap.2012.02.038
P. Sivakumar, R. Ramesh, A. Ramanand, S. Ponnusamy, C.J. Muthamizhchelvan, J. Alloys. Compd. 537, 203 (2012). doi:10.1016/j.jallcom.2012.05.067
X.G. Huang, J. Zhang, S.R. Xiao, G.S. Chen, J. Am. Ceram. Soc. 97(5), 1363–1366 (2014). doi:10.1111/jace.12909
S.R. Jigajeni, M.M. Sutar, S.M. Salunkhe, P.B. Joshi, J. Mater. Sci. Mater. Electron. 23, 1678 (2012). doi:10.1007/s10854-012-0646-4
S. Sharma, V. Singh, R.K. Kotnala, RK Dwivedi, J. Mater. Sci. Mater. Electron. 25, 1915 (2014). doi:10.1007/s10854-014-1820-7
X.G. Huang, J. Zhang, S.R. Xiao, T.Y. Sang, G.S. Chen, Mater Lett. 124, 126–128 (2014). doi:10.1016/j.matlet.2014.03.049
V. Corral-Flores, D. Bueno-Baque´s, R.F. Ziolo, Acta. Mater. 58, 764 (2010). doi:10.1016/j.actamat.2009.09.054
V.V. Shvartsman, F. Alawneh, P. Borisov, Smart. Mater. Struct. 20, 075006 (2011). doi:10.1088/0964-1726/20/7/075006
R.S. Devan, Y.R. Ma, B.K. Chougule, Mater. Chem. Phys. 115, 263 (2009). doi:10.1016/j.matchemphys.2008.11.059
G.V. Duong, R. Groessinger, J. Magn. Magn. Mater. 316, 624 (2007). doi:10.1016/j.matchemphys.2008.11.059
L.V. Leonel, A. Righi, W.N. Mussel, J.B. Silva, N.D.S. Mohallem, Ceram. Int. 37, 1259 (2011). doi:10.1016/j.ceramint.2011.01.017
P.K. Roy, J. Bera, Mater. Chem. Phys. 132(2–3), 354 (2012). doi:10.1016/j.matchemphys.2011.11.031
Acknowledgments
This work was financially supported by the National Natural Science Foundation (51202111), Natural Science Foundation of Jiangsu Province (14KJB430019), Natural Science Foundation of Jiangsu Provincial Universities (BK20141000), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Wang, L. & Zhang, Q. Electromagnetic properties and microwave-absorption properties of BaTiO3/BaZn2Fe16O27 composite in 2–18 GHz. J Mater Sci: Mater Electron 25, 5601–5605 (2014). https://doi.org/10.1007/s10854-014-2349-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-014-2349-5