Abstract
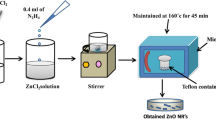
Zinc oxide nanorods have been synthesized by microwave assisted method using zinc nitrate, ethylene glycol and sodium hydroxide as a precursors. The material was characterized by XRD, SEM, EDAX and UV–Visible techniques. XRD analysis revealed all the relevant Bragg’s reflections for wurtzite (hexagonal phase) structure of zinc oxide. The average particle size was obtained 34 nm from the Williamson–Hall plot. The value of particle size determined from XRD was in good agreement with the SEM and TEM results. The direct optical band gap was found to be 3.13 eV.








Similar content being viewed by others
References
R. Baron, F.W. Campbell, I. Streeter, L. Xiao, R.G. Compton, Int. J. Electrochem. Sci. 3, 556 (2008)
A. Bayandori Moghaddam, M. Kazemzad, M.R. Nabid, H.H. Dabaghi, Int. J. Electrochem. Sci. 3, 291 (2008)
D.J. Milliron, S.M. Hughes, Y. Cui, L. Manna, J. Li, L.-W. Wang, A.P. Alivisatos, Nature 430, 190 (2004)
D.C. Look, Mater. Sci. Eng. B 80, 383 (2001)
C.M. Lieber, Solid State Commun. 66, 5309 (1998)
Y. Zhang, K. Suenaga, C. Collies, S. Iijima, Science 281, 973 (1998)
L. Vayssieres, K. Keis, A. Hagfeldt, S.-E. Lindquist, Chem. Mater. 13, 4395 (2001)
Z.W. Pan, Z.R. Dai, Z.L. Wang, Science 292, 1947 (2001)
J.A. Rodriguez, T. Jirsak, J. Dvorak, S. Sambasivan, D.J. Fischer, J. Phys. Chem. B 104, 319 (2000)
W.-C. Shin, M.S. Wu, J. Cryst. Growth 137, 319 (1994)
H.M. Huang, S. Mao, H. Feick, H. Yan, Y. Wu, H. Kind, E. Weber, R. Russo, P.D. Yang, Science 292, 1897 (2001)
N.T. Hung, N.D. Quang, S. Bernik, J. Mater. Res. 16, 2817 (2001)
N.F. Cooray, K. Kushiya, A. Fujimaki, D. Okumura, M. Sato, M. Ooshita, O. Yamase, Jpn. J. Appl. Phys. 38, 6213 (1999)
R. Paneva, D. Gotchev, Sens. Actuat. A: Phys. 72, 79 (1999)
E. Topoglidis, A.E.G. Cass, B. Oregan, J.R. Durrant, J. Electroanal. Chem. 517, 20 (2001)
L. Gao, Q. Li, W.L. Luan, J. Am. Ceram. Soc. 85, 1016 (2002)
C.X. Xu, X.W. Sun, Appl. Phys. Lett. 83, 3806 (2003)
P.X. Gao, Y. Ding, W. Mai, W.L. Hughes, C.S. Lao, Z.L. Wang, Science 309, 1700 (2005)
R.J. Lanf, W.D. Bond, Am. Ceram. Soc. Bull. 63, 278 (1984)
B.S.Shirke, A.A. Patil, P.P.Hankare, K.M.Garadkar, J. Mater. Sci. Mater. Electron. doi: 10.1007/s10854-010-0114-y
E. Ivers-Tiffee, K. Seitz, Am. Ceram. Soc. Bull. 66, 1384 (1987)
J.S. Jie, G.Z. Wang, Q.T. Wang, Y.M. Chen, X.H. Han, P. Wang, J.G. Hou, J. Phys. Chem. B. 108, 11976 (2004)
N.Y. Lee, M.S. Kim, J. Mater. Sci. 26, 1126 (1991)
S.M. Haile, D.W. Johnson, G.H. Wiseman, J. Am. Ceram. Soc. 72, 2004 (1989)
W.J. Li, E.W. Shi, W.Z. Zhong, Z. Yin, J. Cryst. Growth 203, 186 (1999)
B.G. Wang, E.W. Shi, W.Z. Zhong, Cryst. Res. Technol. 33, 937 (1998)
C.H. Lu, C.H. Yeh, Ceram. Int. 26, 351 (2000)
M.C. Neves, T. Trindade, A.M.B. Timmons, J.D. Pedrosa de Jesus, Mater. Res. Bull. 36, 1099 (2001)
J.Q. Hu, Q. Li, N.B. Wong, C.S. Lee, S.T. Lee, Chem. Mater. 14, 1216 (2002)
Y. Sun, G.M. Fuge, N.A. Fox, D.J. Riley, M.N.R. Ashfold, Adv. Mater. 17, 2477 (2005)
Z. Gui, J. Liu, Z.Z. Wang, L. Song, Y. Hu, W.C. Fan, D.Y. Chen, J. Phys. Chem. B 109, 1113 (2005)
Y.H. Ni, X.W. Wei, X. Ma and J.M. Hong. J. Cryst. Growth 283:48 (2005). Compounds. 77: 491 (2010)
F. Gu, S.F. wang, M.K. Lu, G.J. Zhou, D. Xu, D.R. Langmuir, J. Sci. I.R.Iran 20, 3528 (2004)
Acknowledgments
One of the authors (KMG) thankful to UGC for the financial support under the Major Research Project [F.: 35- 337/09 (SR)]. Authors are thankful to Director, UGC-DAE, Indore for providing the TEM facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghule, L.A., Shirke, B.S., Sapnar, K.B. et al. Preparation of zinc oxide nanorods by microwave assisted technique using ethylene glycol as a stabilizing agent. J Mater Sci: Mater Electron 22, 1120–1123 (2011). https://doi.org/10.1007/s10854-010-0270-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-010-0270-0