Abstract
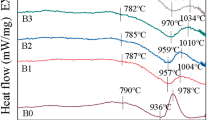
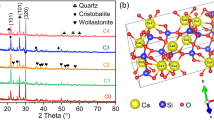
The influences of addition of ZnO on sintering, crystallization behavior and properties of cordierite-based glass-ceramics were investigated. Results show that with increasing ZnO content, the batch melting temperature, glass transition temperature and crystallization temperature all decrease. Addition of ZnO can greatly improve the sinterability of the glass powders and alter the type of the crystalline phases. Addition of 1.5 wt% ZnO seems to be reasonable. Thermal expansion coefficients (TCEs) of samples increase with increasing ZnO contents. The density was found to be an important factor affecting the dielectric loss of the samples. Dielectric constant and TCEs of the sintered bulk samples were found to depend on their relative densities and crystalline phases. The samples doped with 1.5–3.0 wt% ZnO sintered at 950 °C has a low dielectric constant (5.0–5.2), a low dielectric loss (≤0.0018) and a low thermal expansion coefficient (3.6–4.8 × 10−6 K−1), which are promising electronic packaging materials.




Similar content being viewed by others
References
A.H. Kumar, P.W. McMillan, R.R. Tummala, US Pat. 4301324, November 1981
S.H. Knickerbocker, A.H. Kumar, L.W. Herron, Am. Ceram. Soc. Bull. 72, 90 (1993)
R.R. Tummala, J. Am. Ceram. Soc. 74, 905 (1991)
Y. Shimade, K. Utsumi, M. Suzuki, H. Takamizawa, M. Nitta, T. Watari, IEEE Trans. Compon. Hybrids Manuf. Technol. 6, 382 (1983)
D.R. Bridged Holland, P.W. McMillan, Glass Technol. 26, 286 (1985)
H.K. Sarah, H.K. Ananda, L.W. Herron, Am. Ceram. Soc. Bull. 72, 90 (1993)
M. Okuyama, T. Fukui, C. Sakurai, J. Mater. Sci. 28, 4465 (1993)
Mei S., Yang J., Ferreira J.M.F, Meter. Lett. 47, 205 (2001)
W. Zdaniewski, J. Am. Ceram. Soc. 58, 163 (1975)
S.B. Sohn, S.Y. Choi, J. Non-Cryst. Solids 282, 221 (2001)
Y. Hu, H.T. Tsai, J. Mater. Sci. 36, 123 (2001)
C.F. Yang, C.M. Cheng, Ceram. Int. 25, 383 (1999)
G.H. Chen, X.Y. Liu, J. Mater. Sci: Mater. Elelc. 15, 595 (2004)
C. Wang, W. Xu, J. Non-Cryst. Solids 80, 237 (1986)
A.A. Omar, A.W.A. El-Shennavi, A.R. El-Ghannam, J. Mater. Sci. 26, 6049 (1991)
Northwest Institute of Light Industry, Glass Technology (in Chinese) (China Light Industry Press, Beijing, 1982)
J.H. Jean, T.K. Gupta, J. Am. Ceram. Soc. 76, 2010 (1993)
A.A. EI-Kheshen, Br. Ceram. Trans. 102, 205 (2003)
L.S. Chen, S.L. Fu, Jpn. J. Appl. Phys. 31, 3917 (1992)
S.J. Penn, N.M. Alford, A. Templeton, J. Am. Ceram. Soc. 80, 1885 (1997)
L.R. Pinckney, J. Non-Cryst. Solids 255, 171 (1999)
Acknowledgement
This work was supported by the Natural Science Foundation of Guangxi Province, China, under contract No.: 0339066.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Gh. Effect of ZnO addition on properties of cordierite-based glass-ceramics. J Mater Sci: Mater Electron 18, 1253–1257 (2007). https://doi.org/10.1007/s10854-007-9283-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-007-9283-8