Abstract
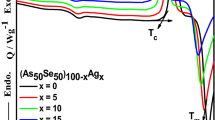
In this paper, bulk glasses with composition of Ag x (As0.33S0.335Se0.335)100-x (x = 0–22 at. %) were investigated. Amorphous structure of samples was confirmed by X-ray diffraction analysis. The structure was deduced from Raman spectra measured for all silver contents in As-S-Se matrix. The thermal properties (T g – glass-forming temperature, T s – softening temperature, T c – temperature of crystallization, T m – melting temperature and C p – specific heat capacity), were obtained from modulated differential scanning calorimetry (MDSC) and thermomechanical analysis (TMA). Optical properties were measured by spectral ellipsometric spectroscopy. Refractive index increasing toward higher silver concentration has been shown either. The value of this refractive index difference (Δn) between As33S33.5Se33.5 and Ag22(As0.33S0.335Se0.335)78 is about 0.3.


Similar content being viewed by others
References
A.V. Kolobov, Photo-induced Metastability in Amorphous Semiconductors. (Wiley-VCH, Weinheim, 2003)
S. Noach, M. Manevich, N.P. Eisenberg, D. Davidov, M. Klebanov, V. Lyubin, Opt. Mater. 28, 1054 (2006)
M. Krbal, T. Wagner, Mil. Vlcek, Mir. Vlcek, M. Frumar, J. Non-Cryst. Solids 352, 2662 (2006)
K. Tanaka, Encylopedia of Materials: Science and Technology (Elsevier Science, Amsterdam, 2000)
T. Wagner, M. Frumar, Mir. Vlcek, S.O. Kasap, Mil. Vlcek, J. Inorg. Mater. 3, 497 (2001)
E. Bychkov, D.L. Price, C.J. Benmore, A.C. Hannon, Solid State Ionics 154–155, 349 (2002)
J. Orava, T. Wagner, M. Krbal, T. Kohoutek, Mil. Vlcek, M. Frumar, J. Non Cryst. Solids 352, 1637 (2006)
W.L. Smith, in Handbook of Laser Science and Technology, vol. 3 part 1, ed by M. J. Weber (Chemical Rubber Co., Boca Raton, 1986), p. 259
H. Ticha, L. Tichy, J. Optoel. Adv. Mat. 4, 381 (2002)
M. Itoh, J. Non-Cryst. Solids 210, 178(1997)
S.R. Elliott, Nature 354, 445 (1991)
S.R. Elliott, Physics of Amorphous Materials 2nd edn. (Longman, Essex, 1990) pp 71)
D. Adler, Amorphous Semiconductors. Butterworths, London (1971)
T. Wagner, Assoc. Prof. Dissertation, (University of Pardubice, 2000)
G. Lucovsky, R.M. Martin, J. Non-Cryst. Solids 8-10, 185 (1972)
H. Hrabalikova, Diploma thesis, (University of Pardubice, Pardubice 2003)
A. Kolobov, H. Oyanagy, A. Roy, K. Tanaka, J. Non-Cryst. Solids 710, 227 (1998)
A. Sklenar, Diploma Thesis, (University of Pardubice, Pardubice, 1997)
J. Schottmiller, M. Tabak, G. Lucovsky, A. Ward, J. Non-Cryst. Solids 4, 80 (1970)
T. Wagner, V. Perina, A. Mackova, Mir. Vlcek, S.O. Kasap, Mil. Vlcek, M. Frumar, Solid State Ionics 141–142, 387 (2001)
V.Yu. Slivka, Yu.M. Vysocanskij, V.A. Stefanovic, V.S. Gerasimenko, D.V. Cepur, Sov. Phys. Sol. Stat. 24, 696 (1982)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krbal, M., Stehlιk, S., Wagner, T. et al. Properties and structure of Ag x (As0.33S0.335Se0.335)100−x bulk glasses. J Mater Sci: Mater Electron 18 (Suppl 1), 213–216 (2007). https://doi.org/10.1007/s10854-007-9200-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-007-9200-1