Abstract
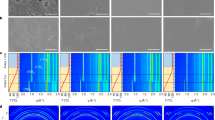
Identifying the structure of iodoplumbate complexes in various solutions is essential for linking the coordination environment of the perovskite precursor to its final properties. In this study, we investigate the structure of methylammonium lead iodide (MAPbI3) perovskite precursor solutions as a function of the volume ratio of N,N-dimethylformamide and dimethyl sulfoxide (DMSO) using X-ray absorption fine structure spectroscopy. It was demonstrated that DMSO modifies the precursor structure at the atomic level. X-ray diffraction, scanning electron microscopy, and UV–Vis spectroscopy analyses showed that the coordination environment of iodoplumbate complexes in precursor solutions is strongly correlated with the morphology of perovskite films. The appropriate amount of DMSO in the precursor solution modifies the Pb-O and Pb-I coordination of iodoplumbate complexes, which could change the growth process of high-crystallization perovskite films. This study sheds light on the formation mechanism of perovskite films from precursor solutions, which will lead to the development of high-quality films and ultimately high-efficiency devices.








Similar content being viewed by others
References
Gharibzadeh S, Hossain IM, Fassl P, Nejand BA, Abzieher T, Schultes M, Ahlswede E, Jackson P, Powalla M, Schäfer S, Rienäcker M, Wietler T, Peibst R, Lemmer U, Richards BS, Paetzold UW (2020) 2D/3D heterostructure for semitransparent perovskite solar cells with engineered bandgap enables efficiencies exceeding 25% in four-terminal tandems with silicon and CIGS. Adv Func Mater 30(19):1909919. https://doi.org/10.1002/adfm.201909919
Mazzarella L, Lin YH, Kirner S, Morales-Vilches AB, Korte L, Albrecht S, Crossland E, Stannowski B, Case C, Snaith HJ, Schlatmann R (2019) Infrared light management using a nanocrystalline silicon oxide interlayer in monolithic perovskite/silicon heterojunction tandem solar cells with efficiency above 25%. Advanced Energy Materials 9(14):1803241. https://doi.org/10.1002/aenm.201803241
Haider SZ, Anwar H, Wang M (2019) Theoretical device engineering for high‐performance perovskite solar cells using CuSCN as hole transport material boost the efficiency above 25%. Physica Status Solidi (a) 216 (11):1900102. doi:https://doi.org/10.1002/pssa.201900102
Kim DH, Muzzillo CP, Tong J, Palmstrom AF, Larson BW, Choi C, Harvey SP, Glynn S, Whitaker JB, Zhang F, Li Z, Lu H, van Hest MFAM, Berry JJ, Mansfield LM, Huang Y, Yan Y, Zhu K (2019) Bimolecular additives improve wide-band-gap perovskites for efficient tandem solar cells with CIGS. Joule 3(7):1734–1745. https://doi.org/10.1016/j.joule.2019.04.012
NREL chart. https://www.nrel.gov/pv/cell-efficiency.html
Papavassiliou GC, Patsis AP, Lagouvardos DJ, Koutselas IB (1993) Spectroscopic studies of (C10H21NH3)2PbI4, (CH3NH3)(C10H21NH3)2Pb2I7, (CH3NH3)PbI3, and similar compounds. Synth Met 57(1):3889–3894. https://doi.org/10.1016/0379-6779(93)90530-A
Hirasawa M, Ishihara T, Goto T, Uchida K, Miura N (1994) Magnetoabsorption of the lowest exciton in perovskite-type compound (CH3NH3)PbI3.Physica B: Condensed Matter (201):427–430. doi:https://doi.org/10.1016/0921-4526(94)91130-4.
Zhao Y, Zhu K (2014) Solution chemistry engineering toward high-efficiency perovskite solar cells. J Phys Chem Lett 5(23):4175–4186. https://doi.org/10.1021/jz501983v
Jung M, Ji SG, Kim G, Seok SI (2019) Perovskite precursor solution chemistry: from fundamentals to photovoltaic applications. Chem Soc Rev 7(48):2011–2038. https://doi.org/10.1039/c8cs00656c
Tan ZK, Moghaddam RS, Lai ML, Docampo P, Higler R, Deschler F, Price M, Sadhanala A, Pazos LM, Credgington D, Hanusch F, Bein T, Snaith HJ, Friend RH (2014) Bright light-emitting diodes based on organometal halide perovskite. Nat Nanotechnol 9(9):687–692. https://doi.org/10.1038/nnano.2014.149
Dou L, Yang YM, You J, Hong Z, Chang WH, Li G, Yang Y (2014) Solution-processed hybrid perovskite photodetectors with high detectivity. Nat Commun 5:5404. https://doi.org/10.1038/ncomms6404
Park N-G (2013) Organometal perovskite light absorbers toward a 20% efficiency low-cost solid-state mesoscopic solar cell. J Phys Chem Lett 4(15):2423–2429. https://doi.org/10.1021/jz400892a
Deschler F, Price M, Pathak S, Klintberg LE, Jarausch D-D, Higler R, Hüttner S, Leijtens T, Stranks SD, Snaith HJ, Atatüre M, Phillips RT, Friend RH (2014) High photoluminescence efficiency and optically pumped lasing in solution-processed mixed halide perovskite semiconductors. J Phys Chem Lett 5(8):1421–1426. https://doi.org/10.1021/jz5005285
Kamat PV (2014) Organometal halide perovskites for transformative photovoltaics. J Am Chem Soc 136(10):3713–3714. https://doi.org/10.1021/ja501108n
Chen Q, Zhou H, Hong Z, Luo S, Duan HS, Wang HH, Liu Y, Li G, Yang Y (2014) Planar heterojunction perovskite solar cells via vapor-assisted solution process. J Am Chem Soc 136(2):622–625. https://doi.org/10.1021/ja411509g
Jeon NJ, Noh JH, Kim YC, Yang WS, Ryu S, Seok SI (2014) Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nat Mater 13(9):897–903. https://doi.org/10.1038/nmat4014
Ahn N, Son DY, Jang IH, Kang SM, Choi M, Park NG (2015) Highly Reproducible Perovskite Solar Cells With Average Efficiency of 18.3% and Best Efficiency of 19.7% Fabricated via Lewis Base Adduct of Lead(II) Iodide. J Am Chem Soc 137 (27):8696–8699. https://doi.org/10.1021/jacs.5b04930
Singh R, Suranagi SR, Kumar M, Shukla VK (2017) Investigations on the role of mixed-solvent for improved efficiency in perovskite solar cell. J Appl Phys 122(23):235302. https://doi.org/10.1063/1.4998630
Hamill JC, Schwartz J, Loo Y-L (2017) Influence of solvent coordination on hybrid organic-inorganic perovskite formation. ACS Energy Lett 3(1):92–97. https://doi.org/10.1021/acsenergylett.7b01057
Yan K, Long M, Zhang T, Wei Z, Chen H, Yang S, Xu J (2015) Hybrid halide perovskite solar cell precursors: colloidal chemistry and coordination engineering behind device processing for high efficiency. J Am Chem Soc 137(13):4460–4468. https://doi.org/10.1021/jacs.5b00321
Sharenko A, Mackeen C, Jewell L, Bridges F, Toney MF (2017) Evolution of Iodoplumbate complexes in methylammonium lead iodide perovskite precursor solutions. Chem Mater 29(3):1315–1320. https://doi.org/10.1021/acs.chemmater.6b04
Stamplecoskie KG, Manser JS, Kamat PV (2015) Dual nature of the excited state in organic–inorganic lead halide perovskites. Energy Environ Sci 8(1):208–215. https://doi.org/10.1039/c4ee02988g
Hamill JC, Romiluyi O, Thomas SA, Cetola J, Schwartz J, Toney MF, Clancy P, Loo Y-L (2020) Sulfur-donor solvents strongly coordinate Pb2+ in hybrid organic-inorganic perovskite precursor solutions. J Phys Chem C 124(27):14496–14502. https://doi.org/10.1021/acs.jpcc.0c03465
Chu S, Zheng L, An P, Gong H, Hu T, Xie Y, Zhang J (2017) Time-resolved XAFS measurement using quick-scanning techniques at BSRF. J Synch Rad 24(3):674–678. https://doi.org/10.1107/s1600577517005276
Ravel B, Newville M (2005) ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J Synch Rad 12(4):537–541. https://doi.org/10.1107/s0909049505012719
Wakamiya A, Endo M, Sasamori T, Tokitoh N, Ogomi Y, Hayase S, Murata Y (2014) Reproducible fabrication of efficient perovskite-based solar cells: x-ray crystallographic studies on the formation of CH3NH3PbI3 layers. Chem Lett 43(5):711–713. https://doi.org/10.1246/cl.140074
cLeod JA, Wu Z, Sun B, Liu L (2016) The influence of the I/Cl ratio on the performance of CH3NH3PbI(3–x)Cl(x)-based solar cells: why is CH3NH3I : PbCl2 = 3: 1 the “magic” ratio? Nanoscale 8(12):6361–6368. https://doi.org/10.1039/c5nr06217a
Persson I, Lyczko K, Lundberg D, Eriksson L, Placzek A (2011) Coordination chemistry study of hydrated and solvated lead(II) ions in solution and solid state. Inorg Chem 50(3):1058–1072. https://doi.org/10.1021/ic1017714
Gao L, Zhang F, Xiao C, Chen X, Larson BW, Berry JJ, Zhu K (2019) Improving Charge Transport via Intermediate-Controlled Crystal Growth in 2D Perovskite Solar Cells. Adv Func Mater 29(47):1901652. https://doi.org/10.1002/adfm.201901652
Mei AY, Li X, Liu LF, Ku ZL, Liu TF, Rong YG, Xu M, Hu M, Chen JZ, Yang Y, Gratzel M, Han HW (2014) Science 345:295–298. https://doi.org/10.1126/science.1254763
Runa A, Feng S, Wen G, Feng F, Wang J, Liu L, Su P, Yang H, Fu W (2017) Highly reproducible perovskite solar cells based on solution coating from mixed solvents. J Mater Sci 53(5):3590–3602. https://doi.org/10.1007/s10853-017-1842-7
Cai B, Zhang W-H, Qiu J (2015) Solvent engineering of spin-coating solutions for planar-structured high-efficiency perovskite solar cells. Chin J Catal 36(8):1183–1190. https://doi.org/10.1016/s1872-2067(15)60929-9
Li B, Li M, Fei C, Cao G, Tian J (2017) Colloidal engineering for monolayer CH3NH3PbI3 films toward high performance perovskite solar cells. J Mater Chem A 5(46):24168–24177. https://doi.org/10.1039/c7ta08761f
Acknowledgement
This work was financially supported by the National Key Research and Development Program of China (grant No.2017YFA0403400) and the National Natural Science Foundation of China (NSFC) (U1932201, grant No.11705225).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Mark Bissett.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zuo, S., Chu, S., An, P. et al. Solvent coordination engineering for high-quality hybrid organic-inorganic perovskite films. J Mater Sci 56, 9903–9913 (2021). https://doi.org/10.1007/s10853-021-05870-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-05870-w