Abstract
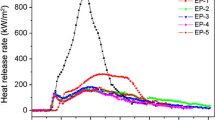
Despite the vast available literature on the synergistic action between zeolites and intumescent formulations, the influence of the acidity of the zeolite on the flame-retardant properties of the materials has not yet been properly addressed. This work investigates the effect of the concentration and the strength of the acidic sites of faujasite Y zeolites on their synergistic action with an intumescent formulation composed of ammonium polyphosphate (APP) and pentaerythritol (PER) in a polypropylene matrix. The results from the limiting oxygen index, cone calorimetry and glow-wire indicate that the zeolites with higher concentration of moderate strength acidic sites can catalyse more efficiently the reaction between APP and PER, which produces phosphate esters, precursors of char, enhancing the flame-retardant properties. However, an over increase in the acidic sites strength shows the opposite effect, as the zeolite can be prematurely deactivated during the initial steps of char formation. Therefore, the increase in the concentration of the faujasite Y acidic sites with moderate strength might be a good strategy in order to obtain materials with better flame-retardant properties.







Similar content being viewed by others
References
Legler J (2003) Are brominated flame retardants endocrine disruptors? Environ Int 29:879–885. https://doi.org/10.1016/S0160-4120(03)00104-1
Lewin M (1998) Physical and chemical mechanisms of flame retarding of polymers. In: Le Bras M, Camino G et al (eds) Fire retardancy of polymers. The use of intumescence. The Real Society of Chemistry, London, pp 3–32
Estevão LRM, Le Bras M, Delobel R, Nascimento RSV (2005) Spent refinery catalyst as a synergistic agent in intumescent formulations: influence of the catalyst’s particle size and constituents. Polym Degrad Stab 88:444–455. https://doi.org/10.1016/j.polymdegradstab.2004.11.016
Bourbigot S, Duquesne S (2007) Fire retardant polymers: recent developments and opportunities. J Mater Chem 17:2283. https://doi.org/10.1039/b702511d
Donmez Cavdar A, Boran Torun S, Ertas M, Mengeloglu F (2018) Ammonium zeolite and ammonium phosphate applied as fire retardants for microcrystalline cellulose filled thermoplastic composites. Fire Saf J. https://doi.org/10.1016/j.firesaf.2018.11.008
Bourbigot S, Bras M Le, Bréant P et al (1996) Zeolites: new synergistic agents for intumescent fire retardant thermoplastic formulations—criteria for the choice of the zeolite. Fire Mater 20:145–154. https://doi.org/10.1002/(SICI)1099-1018(199605)20:3<145::AID-FAM569>3.0.CO;2-L
Bourbigot S, Le Bras M, Delobel R et al (1996) Synergistic effect of zeolite in an intumescence process: study of the carbonaceous structures using solid-state NMR. J Chem Soc Faraday Trans 92:149. https://doi.org/10.1039/ft9969200149
Bourbigot S, Le Bras M, Delobel R, Trémillon J-M (1996) Synergistic effect of zeolite in an intumescence process. Study of the interactions between the polymer and the additives. J Chem Soc Faraday Trans 92:3435–3444. https://doi.org/10.1039/FT9969203435
Ben Mya O, Bita M, Louafi I, Djouadi A (2018) Esterification process catalyzed by ZSM-5 zeolite synthesized via modified hydrothermal method. MethodsX 5:277–282. https://doi.org/10.1016/j.mex.2018.03.004
Kirumakki SR, Nagaraju N, Chary KVR (2006) Esterification of alcohols with acetic acid over zeolites Hβ, HY and HZSM5. Appl Catal A Gen 299:185–192. https://doi.org/10.1016/j.apcata.2005.10.033
Marchetti JM, Errazu AF (2008) Comparison of different heterogeneous catalysts and different alcohols for the esterification reaction of oleic acid. Fuel 87:3477–3480. https://doi.org/10.1016/j.fuel.2008.05.011
Wang J, Yang K, Zheng X (2009) Studies on the effect of 4A zeolite on the properties of intumescent flame-retardant agent filled natural rubber composites. J Polym Res 16:427–436. https://doi.org/10.1007/s10965-008-9245-8
Camino G, Costa L, Trossarelli L et al (1985) Study of the mechanism of intumescence in fire retardant polymers: part VI-mechanism of ester formation in ammonium polyphosphate-pentaerythritol mixtures. Polym Degrad Stab 12:213–228. https://doi.org/10.1016/0141-3910(85)90090-4
Bourbigot S, Le Bras M, Delobel R et al (1995) Carbonization mechanisms resulting from intumescence-part II. Association with an ethylene terpolymer and the ammonium polyphosphate-pentaerythritol fire retardant system. Carbon N Y 33:283–294. https://doi.org/10.1016/0008-6223(94)00131-I
Lecouvet B, Sclavons M, Bailly C, Bourbigot S (2013) A comprehensive study of the synergistic flame retardant mechanisms of halloysite in intumescent polypropylene. Polym Degrad Stab 98:2268–2281. https://doi.org/10.1016/j.polymdegradstab.2013.08.024
Ribeiro FR, Guisnet M (2004) Zeólitos - Um Nanomundo ao Serviço da Catálise. Fundação Calouste Gulbenkian, Lisboa
Barrett EP, Joyner LG, Halenda PP (1951) The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Am Chem Soc 73:373–380. https://doi.org/10.1021/ja01145a126
Lippens B (1965) Studies on pore systems in catalysts V. The t method. J Catal 4:319–323. https://doi.org/10.1016/0021-9517(65)90307-6
UNDERWRITERS’ LABORATORIES (2003) Test for Flammability of Plastic Materials for Parts in Devices and Appliances- UL-94. Northbrook
Rawle A (2003) Basic principles of particle size analysis. Surf Coatings Int 86:58–65
Sohn JR, DeCanio SJ, Lunsford JH, O’Donnell DJ (1986) Determination of framework aluminium content in dealuminated Y-type zeolites: a comparison based on unit cell size and wavenumber of i.r. bands. Zeolites 6:225–227. https://doi.org/10.1016/0144-2449(86)90054-0
Li CY, Rees LVC (1986) Thermogravimetric studies of faujasites with different Si Al ratios. Zeolites 6:217–220. https://doi.org/10.1016/0144-2449(86)90052-7
Almeida KA, Hammer P, Cardoso D (2019) Faujasites exchanged with alkylammonium cations applied to basic catalysis. Microporous Mesoporous Mater 282:159–168. https://doi.org/10.1016/j.micromeso.2019.03.024
Hidalgo CV, Itoh H, Hattori T, Niwa M, Murakami Y (1984) Measurement of the acidity of various zeolites by desorption of ammonia. J Catal 85:362–369
Acknowledgements
This work was performed thanks to CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), FAPERJ (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro) and INOMAT (Instituto Nacional de Ciência, Tecnologia e Inovação em Materiais Complexos Funcionais) financial support. This study was financed in part by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) – Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ribeiro, S.P.S., Martins, R.C., Barbosa, G.M. et al. Influence of the zeolite acidity on its synergistic action with a flame-retarding polymeric intumescent formulation. J Mater Sci 55, 619–630 (2020). https://doi.org/10.1007/s10853-019-04047-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-04047-w