Abstract
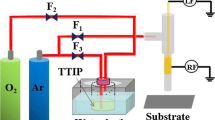
Titanium dioxide has been widely applied in many fields, but the photon utilization efficiency of it was low because of the wide band gap, amounting to 3.2 eV. Many studies have shown that doping TiO2 with nonmetals (N, S, C and so on) could narrow the TiO2 band gap. In the paper, the titanium tetraisopropoxide (TTIP) used as the precursor in the argon/oxygen which was driven by dual-frequency (100 kHz/100 MHz) atmospheric pressure plasma jet via tuning the nitrogen flow ratio was investigated. The structures of the synthetic C, N-codoped TiO2 hollow sphere were investigated by X-ray diffraction and Raman shift spectroscopy. The results indicated that the synthetic crystals consist of anatase and rutile. The scanning electron microscopy and transmission electron microscopy demonstrated that the synthetic crystals were hollow spheres. The elements of the C, N-codoped TiO2 were characterized by energy-disperse spectroscopy. The patterns reveal that carbon and nitrogen are incorporated in the deposited TiO2 crystal. The C element increased but N element decreased with the increase in nitrogen gas flow. The chemical bond and composition of TiO2 were characterized by X-ray photoelectron spectroscopy, which indicates that C is derived from the dissociation of tetraisopropoxide and N element is originated from the electron ionization of N2 molecules. The optical absorption of these samples was detected by UV–Vis absorbance spectra. The results indicated that the band gap of S1 and S2 is 2.91 and 3.10 eV, respectively. Excited species of the plasma discharge were diagnosed by optical emission spectroscopy.








Similar content being viewed by others
References
Chen XB, Mao SS (2007) Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev 107:2891–2959
Lu BA, Zhu CQ, Zhang ZX et al (2012) Preparation of highly porous TiO2 nanotubes and their catalytic applications. J Mater Chem 22:1375–1379
Wang H, Ma D, Huang X et al (2012) General and controllable synthesis strategy of metal oxide/TiO2 hierarchical heterostructures with improved lithium-ion battery performance. Sci Rep 2:136
Hisatomi T, Kubota J, Domen K (2014) Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem Soc Rev 3:7520–7535
Chen Q, Liu Q, Hubert J et al (2017) Deposition of photocatalytic anatase titanium dioxide films by atmospheric dielectric barrier discharge. Surf Coat Technol 310:173–179
Obuchi E, Sakamoto T, Nakano K et al (1999) Photocatalytic decomposition of acetaldehyde over Ti02/Si02 catalyst. Chem Eng Sci 54:1525–1530
Asahi R, Morikawa T, Ohwaki T et al (2001) Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293:269–271
Zou Z, Ye JH, Sayama K et al (2001) Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature 414:625–627
Chimupala Y et al (2014) Synthesis and characterization of a mixed phase of anatase TiO2 and TiO2(B) by low pressure chemical vapour deposition (LPCVD) for high photocatalytic activity. J Phys: Conf Ser 522:012074
Di Paola A, Marci G, Palmisano L et al (2002) Preparation of polycrystalline TiO2 photocatalysts impregnated with various transition metal ions: characterization and photocatalytic activity for the degradation of 4-nitrophenol. J Phys Chem B 106:637–645
Chen X, Lou Y, Samia ACS et al (2005) Formation of oxynitride as the photocatalytic enhancing site in nitrogen-doped titania nanocatalysts: comparison to a commercial nanopower. Adv Func Mater 15:41–49
Wang X, Wang L-L et al (2019) Fabrication and photocatalytic performance of C, N, F-tridoped TiO2 nanotubes. Catal Today 327:182–189
Wang Y, Yuan Q, Yin G (2017) A new method for deposition nitrogen-doped TiO2 nanofibers with mixed phases of anatase and rutile. Surf Interface Anal 49:967–972
Vitiello RP, Macak JM, Ghicov A et al (2006) N-doping of anodic TiO2 nanotubes using heat treatment in ammonia. Electrochem Commun 8:544–548
Di Valentin C, Finazzi E, Pacchioni G et al (2007) N-doped TiO2: theory and experiment. Chem Phys 339:44–56
Devi LG, Kavitha R (2013) A review on non metal ion doped titania for the photocatalytic degradation of organic pollutants under UV/solar light: role of photogenerated charge carrier dynamics in enhancing the activity. Appl Catal B 140:559–587
Hoang S, Berglund SP, Hahn NT et al (2012) Enhancing visible light photo-oxidation of water with TiO2 nanowire arrays via cotreatment with H2 and NH3: synergistic effects between Ti3 + and N. J Am Chem Soc 134:3659–3662
Guo B, Liu Z, Hong L et al (2005) Sol gel derived photocatalytic porous TiO2 thin films. Surf Coat Technol 198:24–29
Kolen’ko YU, Churagulov BR, Kunst M et al (2004) Photocatalytic properties of titania powders prepared by hydrothermal method. Appl Catal B 54:51–58
Sirghi L, Hatanaka Y, Aoki T (2005) Photocatalytic chemisorption of water on titanium dioxide thin films obtained by radio frequency magnetron deposition. Appl Surf Sci 244:408–411
Szymanowski H, Sobczyk A, Gazicki-Lipman M et al (2005) Plasma enhanced CVD deposition of titanium oxide for biomedical applications. Surf Coat Technol 200:1036–1040
Kment S, Kluson P, Zabova H et al (2009) Atmospheric pressure barrier torch discharge and its optimization for flexible deposition of TiO2 thin coatings on various surfaces. Surf Coat Technol 204:667–675
Chang DL, Li XS, Zhao TL et al (2012) Non-thermal effect of atmospheric-pressure RF cold plasma on photocatalytic activity of As-deposited TiO2 film. Chem Vap Deposition 18:121–125
Nie LH, Shi C, Xu Y et al (2007) Atmospheric cold plasmas for synthesizing nanocrystalline anatase TiO2 using dielectric barrier discharges. Plasma Processes Polym 4:574–582
Di LB, Li XS, Shi C et al (2009) Atmospheric-pressure plasma CVD of TiO2 photocatalytic films using surface dielectric barrier discharge. J Phys D Appl Phys 42:032001–032005
Zhou YJ, Yuan QH, Li F et al (2013) Nonequilibrium atmospheric pressure plasma jet using a combination of 50 kHz/2 MHz dual-frequency power sources. Phys Plasmas 20:113502
Wang Y, Yuan QH, Yin GQ et al (2016) Synthesis of mixed-phase TiO2 nanopowders using atmospheric pressure plasma jet driven by dual-frequency power sources. Plasma Chem Plasma Process 36:1471–1484
Yin G, Wang Y, Yuan Q (2018) Ti3+-doped TiO2 hollow sphere with mixed phases of anatase and rutile prepared by dual-frequency atmospheric pressure plasma jet. J Nanopart Res 208:1–12
Ohsaka T, Izumi F, Fujiki Y et al (1978) Raman spectrum of anatase, TiO2. J Raman Spectrosc 7:321–324
Narayanan PS (1950) Raman spectroscopy of Rutilt (TiO2). Proc Indian Acad Sci Sect A 32:279
Montoro LA, Corio P, Rosolen JM (2007) A comparative study of alcohols and ketones as carbon precursor for multi-walled carbon nanotube growth. Carbon 45:1234–1241
Urbonaite S, Hälldahl L, Svensson G (2008) Raman spectroscopy studies of carbide derived carbons. Carbon 46:1942–1947
Wang Y, Yuan Q, Yin G et al (2017) A new method for deposition nitrogen-doped TiO2 nanofibers with mixed phases of anatase and rutile. Surf Interface Anal 49:967–972
Acknowledgements
The authors gratefully acknowledge the support provided by Project of Natural Science Foundation of China (11665021).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yuan, Q., Li, Y. & Yin, G. The synthesis of C, N-codoped TiO2 hollow spheres by a dual-frequency atmospheric pressure cold plasma jet. J Mater Sci 54, 12488–12497 (2019). https://doi.org/10.1007/s10853-019-03804-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03804-1