Abstract
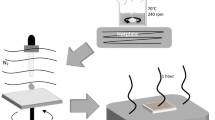
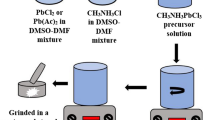
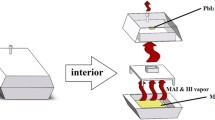
The mixed halide CH3NH3PbI3−xClx crystalline thin film has been prepared by two-step solution-processed repeated dip coating method at an ambient atmosphere. X-ray diffraction study reveals the presence of tetragonal and cubic phases in deposited film. Raman study confirms the metal halide bond in the inorganic framework and organic CH3 stretching/bending of C–H bond in CH3NH3PbI3−xClx perovskite. Scanning electron microscopy shows that cuboid and polyhedral-like crystal grains of 100 nm to 2 μm may find applications in optoelectronics. The perovskite CH3NH3PbI3−xClx thin film shows high spectral absorption coefficient of the order of 106 m−1. In optical band gap study, we found the coexistence of cubic and tetragonal perovskite phases. The energy band gap is dominated by cubic phase having Eg = 2.50 eV over tetragonal phase with band gap Eg = 1.67 eV. The room-temperature photoluminescence study confirms band edge, shallow and deep-level emissions. The temperature-dependent cathodoluminescence study shows red, green and ultraviolet emissions. The dominating green luminescence evolved for cubic phase at 2.51 eV. The red and ultraviolet emissions are also found for mixed-phase CH3NH3PbI3−xClx thin film, suitable for preparation of light-emitting devices.






Similar content being viewed by others
References
Chen Q, De Marco N, Yang Y, Song TB, Chen CC, Zhao H, Hong Z, Zhou H, Yang Y (2015) Under the spotlight: the organic-inorganic hybrid halide perovskite for optoelectronic applications. Nano Today 10:355–396
Huang J, Xiang S, Yu J, Li C-Z (2019) Highly efficient prismatic perovskite solar cells. Energy Environ Sci 12:1265–1273
Nandi P, Giri C, Swain D, Manju U, Topwal D (2019) Room temperature growth of CH3NH3PbCl3 single crystals by solvent evaporation method. CrystEngComm 21:656–661
Zheng E, Yuh B, Tosado GA, Yu Q (2017) Solution-processed visible-blind UV-A photodetectors based on CH3NH3PbCl3 perovskite thin films. J Mater Chem C 5:3796–3806
Pang G, Lan X, Li R, He Z, Chen R (2019) Influence of mixed organic cations on the structural and optical properties of lead tri-iodide perovskites. Nanoscale 11:5215–5221
Ansari MIH, Qurashi A, Nazeeruddin MK (2018) Frontiers, opportunities and challenges in perovskite solar cells: a critical review. J Photochem Photobiol C 35:1–24
Xionga H, Zabihia F, Wanga H, Zhanga Q, Eslamian M (2018) Grain engineering by ultrasonic substrate vibration post treatment of wet perovskite films for annealing-free, high performance, and stable perovskite solar cells. Nanoscale 10:8526–8535
Yakunin S, Shynkarenko Y, Dirin DN, Cherniukh I, Kovalenko MV (2017) Non-dissipative internal optical filtering with solution-grown perovskite single crystals for full-colour imaging. NPG Asia Mater 9:e431
Chin S-H, Choi JW, Woo HC, Kim JH, Lee HS, Lee C-L (2019) Realizing a highly luminescent perovskite thin film by controlling the grain size and crystallinity through solvent vapour annealing. Nanoscale 11:5861–5867
Kim H, Zhao L, Price JS, Grede AJ, Roh K, Brigeman AN, Lopez M, Rand BP, Giebink NC (2018) Hybrid perovskite light emitting diodes under intense electrical excitation. Nat Commun 9:4893
http://www.nrel.gov/pv/assets/images/efficiency-chart.png. Accessed 23 Dec 2018
Stranks SD, Hoye RLZ, Di D, Friend RH, Deschler F (2018) The physics of light emission in halide perovskite devices. Adv Mater. https://doi.org/10.1002/adma.201803336
Ahmadian-Yazdi M-R, Habibi M, Eslamian M (2018) Excitation of wet perovskite films by ultrasonic vibration improves the device performance. Appl Sci 8:308
Ahmadian-Yazdi M-R, Eslamian M (2018) Toward scale-up of perovskite solar cells: annealing-free perovskite layer by low-cost ultrasonic substrate vibration of wet films. Mater Today Commun 14:151–159
Lee JW, Yu H, Lee K, Bae S, Kim J, Han GR, Hwang D, Kim SK, Jang J (2019) Highly crystalline perovskite-based photovoltaics via two-dimensional liquid cage annealing strategy. J Am Chem Soc 141:5808–5814
Lee B, Hwang T, Lee S, Shin B, Park B (2019) Microstructural evolution of hybrid perovskites promoted by chlorine and its impact on the performance of solar cell. Sci Rep 9:4803
Sanches AWP, da Silva MAT, Cordeiro NJA, Urbano A, Lourenço SA (2019) Effect of intermediate phases on the optical properties of PbI2-rich CH3NH3PbI3 organic–inorganic hybrid perovskite. Phys Chem Chem Phys 21:5253–5261
Wang Q, Phung N, Girolamo DD, Vivo P, Abate A (2019) Enhancement in lifespan of halide perovskite solar cells. Energy Environ Sci 12:865–886
Habibi M, Rahimzadeh A, Bennouna I, Eslamian M (2017) Defect-free large-area (25 cm2) light absorbing perovskite thin films made by spray coating. Coatings 7:42
Karim AMMT, Rahman MA, Hossain MS, Khan MKR, Rahman MM, Kamruzzaman M, Ton-That C (2018) Multi-color excitonic emissions in chemical dip-coated organolead mixed-halide perovskite. Chem Sel 3:6525–6530
Adnan M, Lee JK (2018) All sequential dip-coating processed perovskite layers from an aqueous lead precursor for high efficiency perovskite solar cells. Sci Rep 8:2168
Chen J, Wan Z, Liu J, Fu S-Q, Zhang F, Yang S, Tao S, Wang M, Chen C (2018) Growth of compact CH3NH3PbI3 thin films governed by the crystallization in PbI2 matrix for efficient planar perovskite solar cells. ACS Appl Mater Interfaces 10:8649–8658
Wahl KJ, Chromik RR, Lee GY (2008) Quantitative in situ measurement of transfer film thickness by a Newton’s rings method. Wear 264:731–736
Winston AW, Baer CA, Allen LR (2013) A simple film thickness gauge utilizing Newton’s rings. In: Proceedings of the sixth national symposium on vacuum technology transactions, pp 249–254
Raveesha KH, Doddamani VH, Prasad BK (2014) On a method to employ Newton’s rings concept to determine thickness of thin films. Int Lett Chem Phys Astron 22:1–7
Maculan G, Sheikh AD, Abdelhady AL, Saidaminov MI, Haque MA, Murali B, Alarousu E, Mohammed OF, Wu T, Bakr OM (2015) CH3NH3PbCl3 Single crystals: inverse temperature crystallization and visible-blind UV-photodetector. J Phys Chem Lett 6:3781–3786
Yu H, Wang F, Xie F, Li W, Chen J, Zhao N (2014) The role of chlorine in the formation process of “CH3NH3PbI3−xClx” perovskite. Adv Funct Mater 24:7102–7108
Xu Y, Zhu L, Shi J, Lv S, Xu X, Xiao J, Dong J, Wu H, Luo Y, Li D, Meng Q (2015) Efficient hybrid mesoscopic solar cells with morphology-controlled CH3NH3PbI3−xClx derived from two-step spin coating method. ACS Appl Mater Interfaces 7:2242–2248
Luo S, Daoud WA (2016) Crystal structure formation of CH3NH3PbI3−xClx perovskite. Materials 9:123
Chi L, Swainson I, Cranswicka L, Her JH, Stephens P, Knop O (2005) The ordered phase of methylammonium lead chloride CH3ND3PbCl3. J Solid State Chem 178:1376–1385
Dimesso L, Dimamay M, Hamburger M, Jaegermann W (2014) Properties of CH3NH3PbX3 (X = I, Br, Cl) powders as precursors for organic/inorganic solar cells. Chem Mater 26:6762–6770
Sedighi R, Tajabadi F, Shahbazi S, Gholipour S, Taghavinia N (2016) Mixed-halide CH3NH3PbI3−xXx (X = Cl, Br, I) perovskites: vapor-assisted solution deposition and application as solar cell absorbers. Chem Phys Chem 17:2382–2388
Pistor P, Ruiz A, Cabot A, Roca VI (2016) Advanced Raman spectroscopy of methylammonium lead iodide: development of a non-destructive characterisation methodology. Sci Rep 6:35973
Niemann RG, Kontos AG, Palles D, Kamitsos EI, Kaltzoglou A, Brivio F, Falaras P, Cameron PJ (2016) Halogen effects on ordering and bonding of CH3NH3+ in CH3NH3PbX3 (X = Cl, Br, I) hybrid perovskites: a vibrational spectroscopic study. J Phys Chem C 120:2509–2519
Maalej A, Abid Y, Kallel A, Daoud A, Lauti A, Romain F (1997) Phase transitions and crystal dynamics in the cubic perovskite CH3NH3PbCl3. Solid State Commun 103:279–284
Brivio F, Frost JM, Skelton JM, Jackson AJ, Weber OJ, Weller MT, Goni AR, Leguy AMA, Barnes PRF, Walsh A (2015) Lattice dynamics and vibrational spectra of the orthorhombic, tetragonal, and cubic phases of methylammonium lead iodide. Phys Rev B 92:144308
Glaser T, Muller C, Sendner M, Krekeler C, Semonin OE, Hull TD, Yaffe O, Owen JS, Kowalsky W, Pucci A, Lovrincic R (2015) Infrared spectroscopic study of vibrational modes in methylammonium lead halide perovskites. J Phys Chem Lett 6:2913–2918
Dong Q, Yuan Y, Shao Y, Fang Y, Wang Q, Huang J (2015) Abnormal crystal growth in CH3NH3PbI3−xClx using a multi-cycle solution coating process. Energy Environ Sci 8:2464–2470
Xiao L, Xu J, Luan J, Yu X, Zhang B, Dai S, Yao J (2018) Preparation of CH3NH3PbCl3 film with a large grain size using PbI2 as Pb source and its application in photodetector. Mater Lett 220:108–111
Gedamu D, Asuo IM, Benetti D, Basti M, Ka I, Cloutier SG, Rosei F, Nechache R (2018) Solvent-antisolvent ambient processed large grain size perovskite thin films for high-performance solar cells. Sci Rep 8:12885
Moss TS (1959) Optical properties of semiconductor. Academic Press, New York
Karim AMMT, Khan MKR, Rahman MM (2015) Structural and opto-electrical properties of pyrolized ZnO–CdO crystalline thin films. J Semicond 36:053001
Ashaduzzman M, Khan MKR, Karim AMMT, Rahman MM (2018) Influence of chromium on structural, non-linear optical constants and transport properties of CdO thin films. Surf Interfaces 12:135–144
Islam MA, Karim AMMT, Julkarnain M, Badrul AKM, Khan MKR, Khan KA (2017) Opto-transport properties of e-beam evaporated annealed CuInSe2 thin films. Surf Interfaces 8:170–175
Song TB, Chen Q, Zhou H, Jiang C, Wang HH, Yang YM, Liu Y, Youab J, Yang Y (2015) Perovskite solar cells: film formation and properties. J Mater Chem A 3:9032–9050
Tauc J (1974) Amorphous and liquid semiconductors. Plenum Press, New York
Buin A, Pietsch P, Voznyy O, Comin R, Ip AH, Sargent EH, Xu B (2014) Materials processing routes to trap-free halide perovskites. Nano Lett 14:6281–6286
Jiang DS, Jung H, Ploog K (1988) Temperature dependence of photoluminescence from GaAs single and multiple quantum-well heterostructures grown by molecular-beam epitaxy. J Appl Phys 64:1371
Acknowledgements
Authors are thankful to the department of Glass and Ceramic Engineering, Rajshahi University of Engineering & Technology, Bangladesh, for providing XRD and SEM facilities. The authors are also grateful to the Department of Materials Science and Engineering, City University of Hong Kong, for providing Raman spectroscopy measurement. One of the authors M. Azizar Rahman acknowledges the financial support of Australian Government through the Research Training Program Scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karim, A.M.M.T., Hossain, M.S., Khan, M.K.R. et al. Solution-processed mixed halide CH3NH3PbI3−xClx thin films prepared by repeated dip coating. J Mater Sci 54, 11818–11826 (2019). https://doi.org/10.1007/s10853-019-03740-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03740-0