Abstract
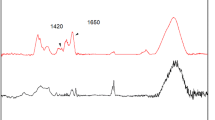
In this work, an enzyme extract immobilization over carbon nanomaterials is presented; that is, carbon nanospheres (CNSs), and multi-walled carbon nanotubes (CNTs), using covalent bonds with glutaraldehyde as a crosslinking compound. Carbon nanostructures were obtained by chemical vapor deposition using an iron–cobalt powder alloy prepared by the sol–gel process and using silica powder as support. Acetylene gas was used as carbon carrier to prepare both the CNTs and CNSs. Both nanostructures were oxidized with an acid treatment in order to add functional groups to the surface and subsequently immobilize the enzymes. Carbon nanostructures were characterized using scanning electron microscopy, infrared spectroscopy and Raman spectroscopy to confirm oxidation, immobilization and structural properties. Electrochemical impedance spectroscopy revealed that carbon nanostructures improve the electron transfer of the electrode. Finally, the cyclic voltammetry provided information about the redox process before and after immobilization. Even more, the catalytic activity of the enzymes extract was evaluated showing that CNSs are the best support.








Similar content being viewed by others
References
Park JH, Xue H, Jung JS, Ryu K (2012) Immobilization of laccase on carbon nanomaterials. Korean J Chem Eng 29:1409–1412. https://doi.org/10.1007/s11814-012-0024-1
Endo M, Hayashi T, Kim Y-A (2006) Large-scale production of carbon nanotubes and their applications. Pure Appl Chem 78:1703–1713. https://doi.org/10.1351/pac200678091703
Vargas-García JR, Calderón-Domínguez G, Ortiz-López J, Manzo-Robledo A, Guadarrama-Fernández L, Chanona-Pérez J, Martínez-Rivas A (2014) Characterization of functionalized multiwalled carbon nanotubes for use in an enzymatic sensor. Microsc Microanal 20:1479–1485. https://doi.org/10.1017/s143192761401304x
Liu M, Zhang F, Li J, Feng Y, Kuang D (2010) Voltammetric determination of bisphenol A in food package by a glassy carbon electrode modified with carboxylated multi-walled carbon nanotubes. Microchim Acta 172:379–386. https://doi.org/10.1007/s00604-010-0512-0
Silva CG, Tavares APM, Dražić G, Silva AMT, Loureiro JM, Faria JL (2014) Controlling the surface chemistry of multiwalled carbon nanotubes for the production of highly efficient and stable laccase-based biocatalysts. Chempluschem 79:1116–1122. https://doi.org/10.1002/cplu.201402054
Oliveira TMBF, Fátima Barroso M, Morais S, De Lima-Neto P, Correia AN, Oliveira MBPP, Delerue-Matos C (2013) Biosensor based on multi-walled carbon nanotubes paste electrode modified with laccase for pirimicarb pesticide quantification. Talanta. 106:137–143. https://doi.org/10.1016/j.talanta.2012.12.017
Kaushik A, Tiwari A, Malhotra BD, Ansari AA, Solanki PR (2009) Multi-walled carbon nanotubes/sol–gel-derived silica/chitosan nanobiocomposite for total cholesterol sensor. Sens Actuators B Chem 137:727–735. https://doi.org/10.1016/j.snb.2008.12.044
Banks CE, Compton RG (2004) i - S E C T I O N E D U C AT I O N Ultrasound: promoting electroanalysis in difficult real world media. Analyst 129:678–683
Mao H, Chen X, Huang R, Chen M, Yang R, Lan P, Zhou M, Zhang F, Yang Y, Zhou X (2018) Fast preparation of carbon spheres from enzymatic hydrolysis lignin: effects of hydrothermal carbonization conditions. Sci Rep 8:2–11. https://doi.org/10.1038/s41598-018-27777-4
Chen C, Yu D, Zhao G, Du B, Tang W, Sun L, Sun Y, Besenbacher F, Yu M (2016) Three-dimensional scaffolding framework of porous carbon nanosheets derived from plant wastes for high-performance supercapacitors. Nano Energy 27:377–389. https://doi.org/10.1016/j.nanoen.2016.07.020
Mahmoud D (2007) Immobilization of invertase by a new economical method using wood sawdust waste. Aust J Appl Sci 1:364–372
Cang-Rong JT, Pastorin G (2009) The influence of carbon nanotubes on enzyme activity and structure: investigation of different immobilization procedures through enzyme kinetics and circular dichroism studies. Nanotechnology. https://doi.org/10.1088/0957-4484/20/25/255102
Liang JF, Li YT, Yang VC (2000) Biomedical application of immobilized enzymes. J Pharm Sci 89:979–990. https://doi.org/10.1002/1520-6017(200008)89:8%3c979:AID-JPS2%3e3.0.CO;2-H
Karim F, Fakhruddin ANM (2012) Recent advances in the development of biosensor for phenol: a review. Rev Environ Sci Biotechnol 11:261–274. https://doi.org/10.1007/s11157-012-9268-9
Kausaite-Minkstimiene A, Mazeiko V, Ramanaviciene A, Ramanavicius A (2011) Evaluation of amperometric glucose biosensors based on glucose oxidase encapsulated within enzymatically synthesized polyaniline and polypyrrole. Sens Actuators B Chem 158:278–285. https://doi.org/10.1016/j.snb.2011.06.019
Feng L, Zhao Y, Luo X, Tu X, Li J, Luo S (2011) Metal chelate affinity to immobilize horseradish peroxidase on functionalized agarose/CNTs composites for the detection of catechol. Sci China Chem 54:1319–1326. https://doi.org/10.1007/s11426-011-4298-7
Andreescu S, Njagi J, Ispas C (2008) Chapter 7 - Nanostructured materials for enzyme immobilization and biosensors, in: V. Erokhin, M.K. Ram, O.B.T.-T.N.F. of O. and C.N. Yavuz (Eds.), Elsevier, Amsterdam, pp 355–394. doi:https://doi.org/10.1016/B978-008045052-0.50009-9
Saifuddin N, Raziah AZ, Junizah AR (2013) Carbon nanotubes: a review on structure and their interaction with proteins. J Chem. https://doi.org/10.1155/2013/676815
Marı R (2017) Characterization of ligninolytic enzyme production in white-rot wild fungal strains suitable for kraft pulp bleaching. https://doi.org/10.1007/s13205-017-0968-2
Pradhan D, Sharon M (2002) Carbon nanotubes, nanofilaments and nanobeads by thermal chemical vapor deposition process. Mater Sci Eng B Solid-State Mater Adv Technol 96:24–28. https://doi.org/10.1016/s0921-5107(02)00309-4
Coville NJ, Mhlanga SD, Nxumalo EN, Shaikjee A (2011) A review of shaped carbon nanomaterials. S Afr J Sci 107:1–15. https://doi.org/10.4102/sajs.v107i3/4.418
Abraham RE, Verma ML, Barrow CJ, Puri M (2014) Suitability of magnetic nanoparticle immobilised cellulases in enhancing enzymatic saccharification of pretreated hemp biomass. Biotechnol Biofuels 7:1–12. https://doi.org/10.1186/1754-6834-7-90
Verma ML, Chaudhary R, Tsuzuki T, Barrow CJ, Puri M (2013) Immobilization of β-glucosidase on a magnetic nanoparticle improves thermostability: application in cellobiose hydrolysis. Bioresour Technol 135:2–6. https://doi.org/10.1016/j.biortech.2013.01.047
Pacley S, Theodore M (2011) Characterization of catalyst effect on carbon nanopearls. NSTI-Nanotech 1:307–310
Domingo C, Santoro G (2007) Espectroscopía Raman de nanotubos de carbono Raman spectroscopy of carbon nanotubes ABSTRACT: Opt Pura Apl 1136:175–186
Malard LM, Pimenta MA, Dresselhaus G, Dresselhaus MS (2009) Raman spectroscopy in graphene. Phys Rep 473:51–87. https://doi.org/10.1016/j.physrep.2009.02.003
Messina G, Donato MG, Santangelo S, Pistone A, Milone C, Galvagno S (2006) Optimisation of gas mixture composition for the preparation of high quality MWCNT by catalytically assisted CVD. Diam Relat Mater 16:1095–1100. https://doi.org/10.1016/j.diamond.2006.12.018
Donato MG, Messina G, Milone C, Pistone A, Santangelo S (2008) Experiments on C nanotubes synthesis by Fe-assisted ethane decomposition. Diam Relat Mater 17:318–324. https://doi.org/10.1016/j.diamond.2007.12.043
Schierz A, Zänker H (2009) Aqueous suspensions of carbon nanotubes: surface oxidation, colloidal stability and uranium sorption. Environ Pollut 157:1088–1094. https://doi.org/10.1016/j.envpol.2008.09.045
Sun X, Li Y (2004) Colloidal Carbon Spheres and Their Core/Shell Structures with Noble-Metal Nanoparticles. Angew Chemie Int Ed 43:597–601. https://doi.org/10.1002/anie.200352386
Ahmed DS, Haider AJ, Mohammad MR (2013) Comparesion of functionalization of multi-walled carbon nanotubes treated by oil olive and nitric acid and their characterization. Energy Proc 36:1111–1118. https://doi.org/10.1016/j.egypro.2013.07.126
Chen J, Xia N, Zhou T, Tan S, Jiang F, Yuan D (2009) Mesoporous carbon spheres: synthesis, characterization and supercapacitance. Int J Electrochem Sci 4:1063–1073
Chauhan N, Pundir CS (2011) An amperometric biosensor based on acetylcholinesterase immobilized onto iron oxide nanoparticles/multi-walled carbon nanotubes modified gold electrode for measurement of organophosphorus insecticides. Anal Chim Acta 701:66–74. https://doi.org/10.1016/j.aca.2011.06.014
Chawla S, Rawal R, Kumar D, Pundir CS (2012) Amperometric determination of total phenolic content in wine by laccase immobilized onto silver nanoparticles/zinc oxide nanoparticles modified gold electrode. Anal Biochem 430:16–23. https://doi.org/10.1016/j.ab.2012.07.025
Mayorga-Martinez CC, Cadevall M, Guix M, Ros J, Merkoçi A (2013) Bismuth nanoparticles for phenolic compounds biosensing application. Biosens Bioelectron 40:57–62. https://doi.org/10.1016/j.bios.2012.06.010
C Jiang, H Chen, C Yu, S Zhang, B Liu, J Kong (2009) Electrochimica Acta Preparation of the Pt nanoparticles decorated poly (N -acetylaniline)/MWNTs nanocomposite and its electrocatalytic oxidation toward formaldehyde, 54: 1134–1140. Doi: 10.1016/j.electacta.2008.08.071
Perenlei G, Tee TW, Yusof NA, Kheng GJ (2011) Voltammetric detection of potassium ferricyanide mediated by multi-walled carbon nanotube/titanium dioxide composite modified glassy carbon electrode. Int J Electrochem Sci 6:520–531
Acknowledegements
Authors show their gratitude to the Michoacan University which provided the biological and electrochemical characterization, especially to Dr. Gerardo Vazquez Marrufo who gave the enzyme extract. We acknowledge the carbon nanomaterials and their structural characterization to CINVESTAV. Mariana Romero Arcos wants to thank the fund CONACYT-SENER for the scholarship support. The M. A. Gonzalez-Reyna and Maria Selene Luna-Martínez’s scholarships were supported by CONACYT under grant 424698.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
We declare that there is no conflict of interests among the authors or with our institution. In fact, our institution is so interested in publishing the results of our different researches.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Romero-Arcos, M., Pérez-Robles, J.F., Guadalupe Garnica-Romo, M. et al. Synthesis and functionalization of carbon nanotubes and nanospheres as a support for the immobilization of an enzyme extract from the mushroom Trametes versicolor. J Mater Sci 54, 11671–11681 (2019). https://doi.org/10.1007/s10853-019-03722-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03722-2