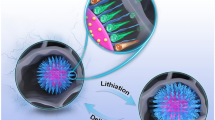
Abstract
We have developed a general methodology to realize surface coating and encapsulation of electrode materials in hierarchical graphite-like carbon matrix and studied how the composition of the copolymer (as the precursor of the graphite-like carbon) and pyrolysis temperature affect the performance of the electrode material. The typical anode material, anatase TiO2, has been used as an example to have encapsulated and distributed the TiO2 nanoparticles in the poly (acrylonitrile-co-styrene) matrix to form hybrid spheres (HSs) Then, the HSs are converted to TiO2/carbon anode materials after appropriate pyrolysis. The copolymers with different ratios of acrylonitrile to styrene from 3:7 to 5:5 have been synthesized, and the pyrolysis is performed in the temperature range from 550 to 850 °C. It is found that both the composition of the copolymer and the pyrolysis temperature can strongly affect the performances of the final TiO2/C anode materials. A proper design optimization is necessary to obtain the electrode materials with the desired performance. Under the optimized conditions, the capacity of the cell with the synthesized TiO2/C as the anode and Li as the reference has reached above 200 mAh g−1 at a current density of 500 mA g−1 and stays stably around 170 mAh g−1 after 1000 cycles.






Similar content being viewed by others
References
Dunn B, Kamath H, Tarascon J-M (2011) Electrical energy storage for the grid: a battery of choices. Science 334:928–935
Chen J (2013) Recent progress in advanced materials for lithium ion batteries. Materials 6:156–183
Li H, Wang Z, Chen L, Huang X (2009) Research on advanced materials for li-ion batteries. Adv Mater 21:4593–4607
Goriparti S, Miele E, De Angelis F, Di Fabrizio E, Proietti Zaccaria R, Capiglia C (2014) Review on recent progress of nanostructured anode materials for Li-ion batteries. J Power Sources 257:421–443
Peled E, Menachem C, Bar-Tow D, Melman A (1996) Improved graphite anode for lithium-ion batteries chemically: bonded solid electrolyte interface and nanochannel formation. J Electrochem Soc 143:L4–L7
Kucinskis G, Bajars G, Kleperis J (2013) Graphene in lithium ion battery cathode materials: a review. J Power Sources 240:66–79
Wu Z-S, Zhou G, Yin L-C, Ren W, Li F, Cheng H-M (2012) Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 1:107–131
Ren J-G, Wu Q-H, Tang H, Hong G, Zhang W, Lee S-T (2013) Germanium-graphene composite anode for high-energy lithium batteries with long cycle life. J Mater Chem A 1:1821–1826
Qin J, Zhang X, Zhao N et al (2015) In situ preparation of interconnected networks constructed by using flexible graphene/Sn sandwich nanosheets for high-performance lithium-ion battery anodes. J Mater Chem A 3:23170–23179
Wang Y, Wei H, Lu Y, Wei S, Wujcik E, Guo Z (2015) Multifunctional carbon nanostructures for advanced energy storage applications. Nanomaterials 5:755–777
Li X, Wang C (2013) Engineering nanostructured anodes via electrostatic spray deposition for high performance lithium ion battery application. J Mater Chem A 1:165–182
Xiang H, Zhang K, Ji G et al (2011) Graphene/nanosized silicon composites for lithium battery anodes with improved cycling stability. Carbon 49:1787–1796
Fan Z-J, Yan J, Wei T et al (2011) Nanographene-constructed carbon nanofibers grown on graphene sheets by chemical vapor deposition: high-performance anode materials for lithium ion batteries. ACS Nano 5:2787–2794
Cao S, Feng X, Song Y et al (2016) In situ carbonized cellulose-based hybrid film as flexible paper anode for lithium-ion batteries. ACS Appl Mater Interfaces 8:1073–1079
Sun B, Chen Z, Kim H-S, Ahn H, Wang G (2011) MnO/C core–shell nanorods as high capacity anode materials for lithium-ion batteries. J Power Sources 196:3346–3349
Liu Z, Lu T, Song T, Yu X-Y, Lou XW, Paik U (2017) Structure-designed synthesis of FeS2@C yolk–shell nanoboxes as a high-performance anode for sodium-ion batteries. Energy Environ Sci 10:1576–1580
Liu N, Lu Z, Zhao J et al (2014) A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nat Nanotechnol 9:187–192
Das S, Li J, Hui R (2015) Simulation of the impact of Si shell thickness on the performance of Si-coated vertically aligned carbon nanofiber as Li-ion battery anode. Nanomaterials 5:2268–2278
Dhanabalan A, Li X, Agrawal R, Chen C, Wang C (2013) Fabrication and characterization of SnO2/graphene composites as high capacity anodes for Li-ion batteries. Nanomaterials 3:606–614
Eftekhari A (2017) Low voltage anode materials for lithium-ion batteries. Energy Storage Mater 7:157–180
Jin L, Zeng G, Wu H, Niederberger M, Morbidelli M (2016) A poly-(styrene-acrylonitrile) copolymer-derived hierarchical architecture in electrode materials for lithium-ion batteries. J Mater Chem A 4:11481–11490
Zhang Y, Tang Y, Li W, Chen X (2016) Nanostructured TiO2-based anode materials for high-performance rechargeable lithium-ion batteries. ChemNanoMat 2:764–775
Su D, Dou S, Wang G (2015) Anatase TiO2: better anode material than amorphous and rutile phases of TiO2 for Na-ion batteries. Chem Mater 27:6022–6029
Bach S, Pereira-Ramos JP, Willman P (2010) Investigation of lithium diffusion in nano-sized rutile TiO2 by impedance spectroscopy. Electrochim Acta 55:4952–4959
Yan D-J, Zhu X-D, Mao Y-C et al (2017) Hierarchically organized CNT@TiO2@Mn3O4 nanostructures for enhanced lithium storage performance. J Mater Chem A 5:17048–17055
Pan L, Liu Y, Xie X, Ye X, Zhu X (2016) Multi-dimensionally ordered, multi-functionally integrated r-GO@TiO2(B)@Mn3O4 yolk–membrane–shell superstructures for ultrafast lithium storage. Nano Res 9:2057–2069
Yan D-J, Zhu X-D, Wang K-X et al (2016) Facile and elegant self-organization of Ag nanoparticles and TiO2 nanorods on V2O5 nanosheets as a superior cathode material for lithium-ion batteries. J Mater Chem A 4:4900–4907
Xu H, Zhu X-D, Sun K-N, Liu Y-T, Xie X-M (2015) Elaborately designed hierarchical heterostructures consisting of carbon-coated TiO2(B) nanosheets decorated with Fe3O4 nanoparticles for remarkable synergy in high-rate lithium storage. Adv Mater Interfaces 2:1500239
Pan L, Zhu X-D, Xie X-M, Liu Y-T (2015) Smart hybridization of TiO2 nanorods and Fe3O4 nanoparticles with pristine graphene nanosheets: hierarchically nanoengineered ternary heterostructures for high-rate lithium storage. Adv Funct Mater 25:3341–3350
Jin L, Wu H, Morbidelli M (2015) Synthesis of water-based dispersions of polymer/TiO2 hybrid nanospheres. Nanomaterials 5:1454–1468
Suresh C, Biju V, Mukundan P, Warrier KGK (1998) Anatase to rutile transformation in sol–gel titania by modification of precursor. Polyhedron 17:3131–3135
Li ZQ, Lu CJ, Xia ZP, Zhou Y, Luo Z (2007) X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 45:1686–1695
Sadezky A, Muckenhuber H, Grothe H, Niessner R, Pöschl U (2005) Raman microspectroscopy of soot and related carbonaceous materials: spectral analysis and structural information. Carbon 43:1731–1742
Zdravkov BD, Čermák JJ, Šefara M, Janků J (2007) Pore classification in the characterization of porous materials: a perspective. Cent Eur J Chem 5:385–395
Beltzung A, Klaue A, Colombo C, Wu H, Storti G, Morbidelli M (2018) Polyacrylonitrile nanoparticle-derived hierarchical structure for CO2 capture. Energy Technol 6:1–11
Acknowledgements
Financial support from the Suisse National Science Foundation (Grant No. 200020_165917) is greatly acknowledged. The research was also supported by the Global Energy Interconnection Research Institute Europe GmbH (Agreement No. SGRIKXJSKF[2017]632).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jin, L., Wu, H. Carbon sphere-based hierarchical architecture for electrode materials: the role of copolymer composition and pyrolysis temperature. J Mater Sci 54, 8226–8235 (2019). https://doi.org/10.1007/s10853-019-03501-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03501-z