Abstract
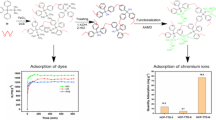
A series of novel silane-based hyper-cross-linked porous polymers was prepared through the Friedel–Crafts alkylation reaction of dimethyldiphenylsilane as the monomer and formaldehyde dimethyl acetal as the external cross-linker. The structures of the polymers were confirmed by FTIR and solid-state cross-polarization magic-angle spinning 13C NMR. We investigated the porous polymers with different ratios of dimethyldiphenylsilane to formaldehyde dimethyl acetal. Porous properties were determined by nitrogen, carbon dioxide and hydrogen adsorptions. The results revealed that the porous materials possessed surface areas from 742 to 1205 m2 g−1 with the increasing amount of formaldehyde dimethyl acetal. Moreover, this material exhibited high thermal stabilities, good CO2 capture, and H2 storage capacities. The material was also used as adsorbent for organic dyes in aqueous solution and showed an excellent adsorption capacity of 952 mg g−1 for Congo red dye. These results suggest that the silane-based hyper-cross-linked porous polymers are promising materials for CO2 capture, H2 storage, and water treatment.









Similar content being viewed by others
References
Li B, Gong R, Wang W, Huang X, Zhang W, Li H, Hu C, Tan B (2011) A new strategy to microporous polymers: knitting rigid aromatic building blocks by external cross-linker. Macromolecules 44:2410–2414
Zhang C, Zhu PC, Tan L, Liu JM, Tan B, Yang XL, Xu HB (2015) Triptycene-based hyper-cross-linked polymer sponge for gas storage and water treatment. Macromolecules 48:8509–8514
Puthiaraj P, Ahn WS (2016) CO2 capture by porous hyper-cross-linked aromatic polymers synthesized using tetrahedral precursors. Ind Eng Chem Res 55:7917–7923
Yue Z, Vakili A (2017) Activated carbon–carbon composites made of pitch-based carbon fibers and phenolic resin for use of adsorbents. J Mater Sci 52:12913–12921. https://doi.org/10.1007/s10853-017-1389-7
Zhang Y, Hsu BYW, Ren C, Li X, Wang J (2015) Silica-based nanocapsules: synthesis, structure control and biomedical applications. Chem Soc Rev 44:315–335
Minceva M, Fajgar R, Markovska L, Meshko V (2008) Comparative study of Zn2+, Cd2+, and Pb2+ removal from water solution using natural clinoptilolitic zeolite and commercial granulated activated carbon. equilibrium of adsorption. Sep Sci Technol 43:2117–2143
Wu D, Xu F, Sun B, Fu R, He H, Matyjaszewski K (2012) Design and preparation of porous polymers. Chem Rev 112:3959–4015
Ben T, Ren H, Ma S et al (2009) Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew Chem Int Ed 48:9457–9460
Kamiya K, Kamai R, Hashimoto K, Nakanishi S (2014) Platinum-modified covalent triazine frameworks hybridized with carbon nanoparticles as methanol-tolerant oxygen reduction electrocatalysts. Nat Commun 5:5040
Geng TM, Zhu H, Song W, Zhu F, Wang Y (2016) Conjugated microporous polymer-based carbazole derivatives as fluorescence chemosensors for picronitric acid. J Mater Sci 51:4104–4114. https://doi.org/10.1007/s10853-016-9732-y
Chen Q, Luo M, HammershØj P, Zhou D, Han Y, Laursen BW, Yan CG, Han BH (2012) Microporous polycarbazole with high specific surface area for gas storage and separation. J Am Chem Soc 134:6084–6087
Luo Y, Li B, Wang W, Wu K, Tan B (2012) Hypercrosslinked aromatic heterocyclic microporous polymers: a new class of highly selective CO2 capturing materials. Adv Mater 24:5703–5707
Fang Q, Wang J, Gu S et al (2015) 3D porous crystalline polyimide covalent organic frameworks for drug delivery. J Am Chem Soc 137:8352–8355
Maly KE (2009) Assembly of nanoporous organic materials from molecular building blocks. J Mater Chem 19:1781–1787
Feng X, Ding X, Jiang D (2012) Covalent organic frameworks. Chem Soc Rev 41:6010–6022
Ding SY, Wang W (2013) Covalent organic frameworks (COFs): from design to applications. Chem Soc Rev 42:548–568
Polak-Krasna K, Dawson R, Holyfield LT, Bowen CR, Burrows AD, Mays TJ (2017) Mechanical characterisation of polymer of intrinsic microporosity PIM-1 for hydrogen storage applications. J Mater Sci 52:3862–3875. https://doi.org/10.1007/s10853-016-0647-4
McKeown NB, Budd PM (2006) Polymers of intrinsic microporosity (PIMs): organic materials for membrane separations, heterogeneous catalysis and hydrogen storage. Chem Soc Rev 35:675–683
Shi Q, Sun H, Yang R, Zhu Z, Liang W, Tan D, Yang B, Li A, Deng W (2015) Synthesis of conjugated microporous polymers for gas storage and selective adsorption. J Mater Sci 50:6388–6394. https://doi.org/10.1007/s10853-015-9191-x
Cooper AI (2009) Conjugated microporous polymers. Adv Mater 21:1291–1295
Xu Y, Jin S, Xu H, Nagai A, Jiang D (2013) Conjugated microporous polymers: design, synthesis and application. Chem Soc Rev 42:8012–8031
Fontanals N, Marcé RM, Borrull F, Cormack PAG (2015) Hypercrosslinked materials: preparation, characterisation and applications. Polym Chem 6:7231–7244
Liu Y, Fan X, Jia X, Zhang B, Zhang H, Zhang A, Zhang Q (2016) Hypercrosslinked polymers: controlled preparation and effective adsorption of aniline. J Mater Sci 51:8579–8592. https://doi.org/10.1007/s10853-016-0118-y
Wood CD, Tan B, Trewin A et al (2007) Hydrogen storage in microporous hypercrosslinked organic polymer networks. Chem Mater 19:2034–2048
Wood CD, Tan B, Trewin A et al (2008) Microporous organic polymers for methane storage. Adv Mater 20:1916–1921
Tsyurupa MP, Davankov VA (2006) Porous structure of hypercrosslinked polystyrene: state-of-the-art mini-review. React Funct Polym 66:768–779
Tan L, Li B, Yang X, Wang W, Tan B (2015) Knitting hypercrosslinked conjugated microporous polymers with external crosslinker. Polymer 70:336–342
Wang S, Tan L, Zhang C, Hussain I, Tan B (2015) Novel POSS-based organic-inorganic hybrid porous materials by low cost strategies. J Mater Chem A 3:6542–6548
Zhu K, Gao Y, Tan X, Chen C (2016) Polyaniline-modified Mg/Al layered double hydroxide composites and their application in efficient removal of Cr(VI). ACS Sustain Chem Eng 4:4361–4369
Sing KSW (1982) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem 54:2201–2218
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquérol J, Siemieniewska T (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem 57:603–619
Li B, Guan Z, Wang W, Yang X, Hu J, Tan B, Li T (2012) Highly dispersed Pd catalyst locked in knitting aryl network polymers for Suzuki-Miyaura coupling reactions of aryl chlorides in aqueous media. Adv Mater 24:3390–3395
Woodward RT, Stevens LA, Dawson R et al (2014) Swellable, water- and acid-tolerant polymer sponges for chemoselective carbon dioxide capture. J Am Chem Soc 136:9028–9035
Li B, Huang X, Liang L, Tan B (2010) Synthesis of uniform microporous polymer nanoparticles and their applications for hydrogen storage. J Mater Chem 20:7444–7450
Huang ZH, Zheng X, Lv W, Wang M, Yang QH, Kang F (2011) Adsorption of lead(II) ions from aqueous solution on low-temperature exfoliated graphene nanosheets. Langmuir 27:7558–7562
Kampalanonwat P, Supaphol P (2010) Preparation and adsorption behavior of aminated electrospun polyacrylonitrile nanofiber mats for heavy metal ion removal. ACS Appl Mater Interfaces 2:3619–3627
Ho YS, McKay G (2000) The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res 34:735–742
Shen Y, Li L, Xiao K, Xi J (2016) Constructing three-dimensional hierarchical architectures by integrating carbon nanofibers into graphite felts for water purification. ACS Sustain Chem Eng 4:2351–2358
Xu J, Liu X, Lowry GV, Cao Z, Zhao H, Zhou JL, Xu X (2016) Dechlorination mechanism of 2,4-dichlorophenol by magnetic MWCNTs supported Pd/Fe nanohybrids: rapid adsorption, gradual dechlorination, and desorption of phenol. ACS Appl Mater Interfaces 8:7333–7342
Asfaram A, Ghaedi M, Azqhandi MHA, Goudarzi A, Dastkhoon M (2016) Statistical experimental design, least squares-support vector machine (LS-SVM) and artificial neural network (ANN) methods for modeling the facilitated adsorption of methylene blue dye. RSC Adv 6:40502–40516
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant No. 51503107), Shandong Province Natural Science Foundation (ZR2012EMM009 and ZR2013EMQ005), the Foundation of Key Laboratory of Pulp and Paper Science and Technology of Ministry of Education/Shandong Province of China (No. KF2016).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fu, S., Yao, J., Yang, Z. et al. Silane-based hyper-cross-linked porous polymers and their applications in gas storage and water treatment. J Mater Sci 53, 10469–10478 (2018). https://doi.org/10.1007/s10853-018-2243-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2243-2