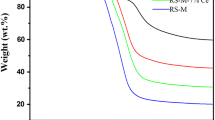
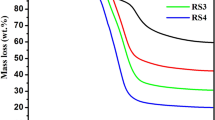
Abstract
A novel resin-based spherical carbon material was successfully prepared by suspension polymerization of alkyl phenol and formaldehyde and steam activation in combination with surface modification by heat treatment with dry air for enhancing Hg0 adsorption. The analysis results demonstrate that the oxidation-modified activated carbon spheres possess better mercury removal performance than untreated sorbents, and the ACS-O300 obtained by oxidation modified at 300 °C is the optimal sorbent at the adsorption temperature range from 25 to 150 °C. The main reason is assigned to the increase of the oxygen functional groups of C=O and C(O)–O–C that play an important role as effective active sites for binding the Hg0, even though the C(O)–O–C predominates in mercury removal performance under higher adsorption temperature. The optimum O2 concentration is 4 vol% at the O2 concentration range from 0 to 8 vol%. SO2 and NO are favorable to the mercury adsorption under 4 vol% O2, while H2O leads to the inhibition of the mercury adsorption. The TPD results suggest that a strong desorption peak at temperature around 235 °C and a weak peak at 324 °C should generate from mercury desorption of C = O and C(O)–O–C, correspondingly. Moreover, the XPS analysis results of the fresh and used sorbent indicates that the C=O and C(O)–O–C serve as strong oxidizer and facilitate electron transfer for converting Hg0 to Hg2+ in the chemisorption process. These results suggest that the obtained resin-based spherical porous carbon (ACS-O300) is promising for Hg0 capture.








Similar content being viewed by others
References
Liu QY, Liu ZY (2013) Carbon supported vanadia for multi-pollutants removal from flue gas. Fuel 108:149–158
Gaffney JS, Marley N (2014) In-depth review of atmospheric mercury: sources, transformations, and potential sinks. Inter J Nanomed 9:1883–1889
Iyyanki V, Muralikrishna VM (2017) Air pollution control technologies. In: Environmental management, Elsevier, India, pp 337–397
Li YT, Yi HH, Tang XL et al (2016) Study on the performance of simultaneous desulfurization and denitrification of Fe3O4–TiO2 composites. Chem Eng J 304:89–97
Tang L, Nagashima KT, Hasegawa K et al (2015) Development of human health damage factors for PM2.5 based on a global chemical transport model. Int J Life Cycle Assess 1–11
Wang SX, Zhang L, Zhao B et al (2012) Mitigation potential of mercury emission form coal-fired power plants in China. Energy Fuels 26:4635–4642
Xu HM, Qu Z, Huang WJ et al (2015) Regenerable Ag/graphene sorbent for elemental mercury capture at ambient temperature. Colloid Surf A 476:83–89
Liu YX, Wang Q, Pan JF et al (2015) A study on removal of elemental mercury in flue gas using fenton solution. J Hazard Mater 292:164–172
Wang FY, Wang SX, Zhang L et al (2016) Characteristics of mercury cycling in the cement production process. J Hazard Mater 302:27–35
Zhao B, Yi HH, Tang XL et al (2016) Copper modified activated coke for mercury removal from coal-fired flue gas. Chem Eng J 286:585–593
Driscoll CT, Mason RP, Chan HM et al (2013) Mercury as a global pollutant: sources, pathways, and effects. Environ Sci Technol 47:4967–4983
Pirrone N, Cinnirella S, Feng X et al (2010) Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos Chem Phys 10:5951–5964
Li GL, Shen BX, Wang SJ et al (2015) Comparative study of elemental mercury removal by three bio-chars from various solid wastes. Fuel 145:189–195
Zhang YS, Duan W, Liu Z et al (2014) Effects of modified fly ash on mercury adsorption ability in an entrained-flow reactor. Fuel 128:274–280
Zhao Y, Hao L, Zhang P et al (2014) An integrative process for Hg0 removal using vaporized H2O2/Na2S2O8. Fuel 13:113–121
Wu H, Liu H, Wang QH et al (2013) Experimental study of homogeneous mercury oxidation under O2/CO2 atmosphere. Proc Combust Inst 34:2847–2854
Li GL, Shen BX, Li BX et al (2015) Elemental mercury removal using biochar pyrolyzed from municipal solid waste. Fuel Process Technol 133:43–50
Ochoa-Gonzalez R, Diaz-Somoano M, Martinez-Tarazona MR (2013) The capture of oxidized mercury from simulated desulphurization aqueous solutions. J Environ Manag 120:55–60
He C, Shen BX, Chen JH et al (2014) Adsorption and oxidation of elemental mercury over Ce–MnOx/Ti-PILCs. Environ Sci Technol 48:7891–7898
Xing L, Xu Y, Zhong Q (2012) Mn and Fe modified fly ash as a superior catalyst for elemental mercury capture under air conditions. Energy Fuels 26:4903–4909
Zhou Q, Duan YF, Hong YG et al (2015) Experimental and kinetic studies of gas-phase mercury adsorption by raw and bromine modified activated carbon. Fuel Process Technol 134:325–332
Xu WQ, Wang HR, Zhu TY et al (2013) Mercury removal from coal combustion flue gas by modified fly ash. J Environ Sci 25:393–398
McLarnon CR, Granite EJ, Pennline HW (2005) The PCO process for photochemical removal of mercury from flue gas. Fuel Process Technol 87:85–89
Zhang Bi XuP, Qiu Y et al (2015) Increasing oxygen functional groups of activated carbon with non-thermal plasma to enhance mercury removal efficiency for flue gases. Chem Eng J 263:1–8
Wang JC, Zhang YP, Han L et al (2013) Simultaneous removal of hydrogen sulfide and mercury from simulated syngas by iron-based sorbents. Fuel 103:73–79
Shen BX, Tian LH, Li FK et al (2017) Elemental mercury removal by the modified bio-char from waste tea. Fuel 272:28–37
Pavlish JH, Hamre LL, Zhuang Y (2010) Mercury control technologies for coal combustion and gasification systems. Fuel 89:838–847
Jun Z, Duan YF, Zhou Q et al (2016) Adsorptive removal of gas-phase mercury by oxygen non-thermal plasma modified activated carbon. Chem Eng J 294:281–289
Zheng Y, Jensen AD, Windelin C et al (2012) Review of technologies for mercury removal from flue gas from cement production processes. Prog Energy Combust Sci 38:599–629
Presto AA, Granite EJ (2006) Survey of catalysts for oxidation of mercury in flue gas. Environ Sci Technol 40:5601–5609
Johnson NC, Manchester S, Sarin L et al (2008) Mercury vapor release from broken compact fluorescent lamps and in situ capture by new nanomaterial sorbents. Environ Sci Technol 42:5772–5778
Saha A, Abram DN, Kuh KP et al (2013) CeO2–TiO2 sorbents for the removal of elemental mercury from syngas. Environ Sci Technol 47:13695–13701
Lee SJ, Seo Y, Lee TG (2004) Removal of gas-phase elemental mercury by iodine and chlorine-impregnated activated carbons. Atmos Environ 38:4887–4893
Mullett M, Pendleto P, Badalyan A (2012) Removal of elemental mercury from Bayer stack gases using sulfur-impregnated activated carbons. Chem Eng J 211:133–142
Ghorishi SB, Keeney RM, Serre SD et al (2002) Development of a Cl-impregnated activated carbon for entrained-flow capture of elemental mercury. Environ Sci Technol 36:4454–4459
Gao Y, Külaots I, Chen X et al (2001) Ozonation for the chemical modification of carbon surfaces in fly ash. Fuel 80:765–768
Chen X, Farber M, Gao Y et al (2003) Mechanisms of surfactant adsorption on non-polar, air-oxidized and ozone-treated carbon surfaces. Carbon 41:1489–1500
Manchester S, Wang XL, Kulaots I et al (2008) High capacity mercury adsorption on freshly ozone-treated carbon surfaces. Carbon 46:518–524
Skodras G, Diamantopoulou I, Sakellaropoulos GP (2007) Role of activated carbon structural properties and surface chemistry in mercury adsorption. Desalination 210:281–286
Li YH, Lee CW, Gullett BK (2003) Importance of activated carbon’s oxygen surface functional groups on elemental mercury adsorption. Fuel 82:451–457
Liu J, Cheney MA, Wu F et al (2011) Effects of chemical functional groups on elemental mercury adsorption on carbonaceous surfaces. J Hazard Mater 186:108–113
Zhang CM, Xie LJ, Song W et al (2013) CO2 Capture with activated carbon grafted by nitrogenous functional groups. Energy Fuels 27:4818–4823
Ludwinowicz J, Jaroniec M (2015) Potassium salt-assisted synthesis of highly microporous carbon spheres for CO2 adsorption. Carbon 82:297–303
Zhang CM, Song W, Sun GH et al (2014) Synthesis, characterization, and evaluation of activated carbon spheres for removal of dibenzothiophene from model diesel fuel. Ind Eng Chem 53:4271–4276
Romero-Anaya AJ, Ouzzine M, Lillo-Ródenas MA et al (2014) Spherical carbons: synthesis, characterization and activation processes. Carbon 68:296–307
Zhao S, Qu Z, Yan N et al (2015) Co-benefit of Ag and Mo for the catalytic oxidation of elemental mercury. Fuel 158:891–897
Zhao S, Xu HM, Mei J et al (2017) Ag–Mo modified SCR catalyst for a co-beneficial oxidation of elemental mercury at wide temperature range. Fuel 200:236–243
Boyano A, Gálvez ME, LázaroFan MJ et al (2006) Characterization and kinetic study of carbon-based briquettes for the reduction of NO. Carbon 44:2399–2403
Shafeeyan MS, Daud WMAW, Houshmand A et al (2011) Ammonia modification of activated carbon to enhance carbon dioxide adsorption: effect of pre-oxidation. Appl Surf Sci 257:3936–3942
Fu KF, Yue QY, Gao BY et al (2013) Preparation, characterization and application of lignin-based activated carbon from black liquor lignin by steam activation. Chem Eng J 228:1074–1082
Aranda A, Murillo R, Garcia T et al (2012) Simulation and optimization of tyre-based steam activated carbons production for gas-phase polycyclic aromatic hydrocarbons abatement. Chem Eng J 187:123–132
Bertrana X, Chollona G, Dentzer J et al (2017) Oxidation behavior at moderate temperature under dry and wet air of phenolic resin-derived carbon. Thermochi acta 649:13–21
Walker PL Jr, Taylor RL, Ranish JM (1991) An update on the carbon–oxygen reaction. Carbon 29:411–421
Terzyk AP (2001) The influence of activated carbon surface chemical composition on the adsorption of acetaminophen (paracetamol) in vitro: Part II. TG, FTIR, and XPS analysis of carbons and the temperature dependence of adsorption kinetics at the neutral pH. Colloids Surf A 177:23–45
Sharma RK, Wooten JB, Baliga VL et al (2004) Characterization of chars from pyrolysis of lignin. Fuel 83:1469–1482
Levi G, Senneca O, Causà M et al (2015) Probing the chemical nature of surface oxides during coal char oxidation by high-resolution XPS. Carbon 90:181–196
Tan Z, Qiu J, Zeng H et al (2015) Removal of elemental mercury by bamboo charcoal impregnated with H2O2. Fuel 90:1471–1475
Tan Z, Sun L, Xiang J et al (2012) Gas-phase elemental mercury removal by novel carbon-based sorbents. Carbon 50:362–371
Moreno-Castilla C, Carrasco-Martin F, Maldonado-Hodar FJ et al (1998) Effects of non-oxidant and oxidant acid treatments on the surface properties of an activated carbon with very low ash content. Carbon 36:145–151
Shen BX, Li GL, Wang FM et al (2015) Elemental mercury removal by the modified bio-char from medicinal residues. Chem Eng J 272:28–37
Zhou ZJ, Liu XW, Zhao B et al (2016) Elemental mercury oxidation over manganese-based perovskite-type catalyst at low temperature. Chem Eng J 288:701–710
Olson ES, Miller SJ, Sharma RK et al (2000) Catalytic effects of carbon sorbents for mercury capture. J Hazard Mater 74:61–79
Liu J, Qu W, Joo SW et al (2012) Effect of SO2 on mercury binding on carbonaceous surfaces. Chem Eng J 184:163–167
Li H, Wu CY, Li Y et al (2011) CeO2–TiO2 catalysts for catalytic oxidation of elemental mercury in low-rank coal combustion flue gas. Environ Sci Technol 45:7394–7400
Ochiai R, Uddin MA, Sasaoka E et al (2009) Effects of HCl and SO2 concentration on mercury removal by activated carbon sorbents in coal-derived flue gas. Energy Fuels 23:4734–4739
Hsi HC, Chen CT (2012) Influences of acidic/oxidizing gases on elemental mercury adsorption equilibrium and kinetics of sulfur-impregnated activated carbon. Fuel 98:229–235
Li Y, Wu CY (2006) Role of moisture in adsorption, photocatalytic oxidation, and reemission of elemental mercury on a SiO2–TiO2 nanocomposite. Environ Sci Technol 40:6444–6448
Li YH, Lee CW, Gullett BK (2002) The effect of activated carbon surface moisture on low temperature mercury adsorption. Carbon 40:65–72
Hutson ND, Attwood BC, Scheckel KG (2007) XAS and XPS characterization of mercury binding on brominated activated carbon. Environ Sci Technol 41:1747–1752
Acknowledgements
This work is financially supported by National Nature Science Foundation of China (Nos. 51002166, 51172251 and 51061130536), National Science Foundation of China for Youths (Nos. 51402324 and 21706179), National Science Foundation of ShanXi for Youths (Nos. 2015021107 and 201701D221037).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, C., Song, W., Zhang, X. et al. Synthesis, characterization and evaluation of resin-based carbon spheres modified by oxygen functional groups for gaseous elemental mercury capture. J Mater Sci 53, 9429–9448 (2018). https://doi.org/10.1007/s10853-018-2231-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2231-6