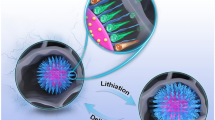
Abstract
Organic–inorganic composites show great potential for organic rechargeable lithium-ion batteries. In this work, two-dimensional phthalocyanine molecules were converted into hybrid nanoparticles with a porous structure and bound to a conductive graphene layer to act as a cathode material. The conductivity of this reduced graphene oxide/Fe-phthalocyanine (rGO/FePc) composite is improved through good interfacial connections and internal polymerization. The FePc spheres were shaped with the assistance of Fe3O4 and immobilized between the layers of reduced graphene oxide (rGO). The electrochemical properties of the organic–inorganic composites were investigated by testing in a lithium-ion cell. A high discharge capacity of 186 mAh g−1 was maintained after 100 cycles at 300 mA g−1, which demonstrates a significant improvement in the cycle life compared to previous reports of phthalocyanine-based electrochemical energy storage behaviour.




Similar content being viewed by others
References
Rogers JA, Someya T, Huang Y (2010) Materials and mechanics for stretchable electronics. Science 80(327):1603–1607. https://doi.org/10.1126/science.1182383
Wu H, Shevlin SA, Meng Q et al (2014) Flexible and binder-free organic cathode for high-performance lithium-ion batteries. Adv Mater 26:3338–3343. https://doi.org/10.1002/adma.201305452
Lee J, Kim H, Park MJ (2016) Long-life, high-rate lithium-organic batteries based on naphthoquinone derivatives. Chem Mater 28:2408–2416. https://doi.org/10.1021/acs.chemmater.6b00624
Zeng R, Xing L, Qiu Y et al (2014) Polycarbonyl(quinonyl) organic compounds as cathode materials for sustainable lithium ion batteries. Electrochim Acta 146:447–454. https://doi.org/10.1016/j.electacta.2014.09.082
Byrne JJ, Driscoll JS, Williams DL (1969) A high energy density lithium/dichloroisocyanuric acid battery system. Electrochem Sci 116:3–5
Liang Y, Tao Z, Chen J (2012) Organic electrode materials for rechargeable lithium batteries. Adv Energy Mater 2:742–769. https://doi.org/10.1002/aenm.201100795
Janoschka T, Hager MD, Schubert US (2012) Powering up the future: radical polymers for battery applications. Adv Mater 24:6397–6409. https://doi.org/10.1002/adma.201203119
Novák P, Müller K, Santhanam KSV, Haas O (1997) Electrochemically active polymers for rechargeable batteries. Chem Rev 97:207–282. https://doi.org/10.1021/cr941181o
Kiya Y, Henderson JC, Hutchison GR, Abruna HD (2007) Synthesis, computational and electrochemical characterization of a family of functionalized dimercaptothiophenes for potential use as high-energy cathode materials for lithium/lithium-ion batteries. J Mater Chem 17:4366–4376. https://doi.org/10.1039/B707235J
Pasquali M, Pistoia G, Boschi T, Tagliatesta P (1987) Redox mechanism and cycling behaviour of nonylbenzo-hexaquinone electrodes in Li cells. Solid State Ionics 23:261–266. https://doi.org/10.1016/0167-2738(87)90003-8
Liu M (1991) Novel solid redox polymerization electrodes. J Electrochem Soc 138:1896. https://doi.org/10.1149/1.2085896
Zhao L, Wang W, Wang A et al (2011) A MC/AQ parasitic composite as cathode material for lithium battery. J Electrochem Soc 158:A991–A996. https://doi.org/10.1149/1.3605719
Bugnon L, Morton CJH, Novak P et al (2007) Synthesis of poly(4-methacryloyloxy-TEMPO) via group-transfer polymerization and its evaluation in organic radical battery. Chem Mater 19:2910–2914. https://doi.org/10.1021/cm063052h
Genorio B, Pirnat K, Cerc-Korosec R et al (2010) Electroactive organic molecules immobilized onto solid nanoparticles as a cathode material for lithium-ion batteries. Angew Chemie - Int Ed 49:7222–7224. https://doi.org/10.1002/anie.201001539
Capone S, Mongelli S, Rella R et al (1999) Gas sensitivity measurements on NO2 sensors based on copper(II) tetrakis(n-butylaminocarbonyl)phthalocyanine LB films. Langmuir 15:1748–1753
O’Regan BC, López-Duarte I, Martínez-Díaz MV et al (2008) Catalysis of recombination and its limitation on open circuit voltage for dye sensitized photovoltaic cells using phthalocyanine dyes. J Am Chem Soc 130:2906–2907. https://doi.org/10.1021/ja078045o
O’Flaherty SM, Hold SV, Cook MJ et al (2003) Molecular engineering of peripherally and axially modified phthalocyanines for optical limiting and nonlinear optics. Adv Mater 15:19–32. https://doi.org/10.1002/adma.200390002
Asai Y, Miyata S, Onishi K et al (2000) Metal-free octacyanophthalocyanine as cathode-active material for a secondary lithium battery. Electrochim Acta 46:77–81. https://doi.org/10.1016/S0013-4686(00)00541-7
Wang Y, Chen J, Jiang C et al (2017) Tetra-β-nitro-substituted phthalocyanines: a new organic electrode material for lithium batteries. J Solid State Electrochem 21:947–954. https://doi.org/10.1007/s10008-016-3419-9
Lee M, Hong J, Kim H et al (2014) Organic nanohybrids for fast and sustainable energy storage. Adv Mater 26:2558–2565. https://doi.org/10.1002/adma.201305005
Ramos-Sanchez G, Callejas-Tovar A, Scanlon LG, Balbuena PB (2014) DFT analysis of Li intercalation mechanisms in the Fe-phthalocyanine cathode of Li-ion batteries. Phys Chem Chem Phys 16:743–752. https://doi.org/10.1039/c3cp53161a
Crowther O, Du LS, Moureau DM et al (2012) Effect of conductive carbon on capacity of iron phthalocyanine cathodes in primary lithium batteries. J Power Sources 217:92–97. https://doi.org/10.1016/j.jpowsour.2012.06.003
Yamaki J (1982) Phthalocyanine cathode materials for secondary lithium cells. J Electrochem Soc 129:5. https://doi.org/10.1149/1.2123792
Chen J, Zhang Q, Zeng M et al (2016) Carboxyl-conjugated phthalocyanines used as novel electrode materials with high specific capacity for lithium-ion batteries. J Solid State Electrochem 20:1285–1294. https://doi.org/10.1007/s10008-016-3126-6
Voiry D, Yang J, Kupferberg J et al (2016) High-quality graphene via microwave reduction of solution-exfoliated graphene oxide. Science 80(353):1413–1416. https://doi.org/10.1126/science.aah3398
Iqbal MZ, Abdala AA, Mittal V et al (2016) Processable conductive graphene/polyethylene nanocomposites: effects of graphene dispersion and polyethylene blending with oxidized polyethylene on rheology and microstructure. Polym (United Kingdom) 98:143–155. https://doi.org/10.1016/j.polymer.2016.06.021
Lin D, Liu Y, Liang Z et al (2016) Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nat Nanotechnol 11:626–632. https://doi.org/10.1038/nnano.2016.32
Georgakilas V, Tiwari JN, Kemp KC et al (2016) Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem Rev 116:5464–5519. https://doi.org/10.1021/acs.chemrev.5b00620
Marcano DC, Kosynkin DV, Berlin JM et al (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814. https://doi.org/10.1021/nn1006368
Guo C, Zhou L, Lv J (2013) Effects of expandable graphite and modified ammonium polyphosphate on the flame-retardant and mechanical properties of wood flour-polypropylene composites. Polym Polym Compos 21:449–456. https://doi.org/10.1002/app
He D-X, Qiu Y, Li L-L et al (2015) Large-scale solvent-thermal synthesis of graphene/magnetite/conductive oligomer ternary composites for microwave absorption. Sci China Mater 58:566–573. https://doi.org/10.1007/s40843-015-0065-y
Zhou K, Zhu Y, Yang X, Li C (2010) One-pot preparation of graphene/Fe3O4 composites by a solvothermal reaction. New J Chem 34:2950. https://doi.org/10.1039/c0nj00283f
Zheng J, Liu ZQ, Zhao XS et al (2012) One-step solvothermal synthesis of Fe3O4@C core–shell nanoparticles with tunable sizes. Nanotechnology 23:165601. https://doi.org/10.1088/0957-4484/23/16/165601
Xuan S, Wang YXJ, Yu JC, Leung KCF (2009) Tuning the grain size and particle size of superparamagnetic Fe3O4 microparticles. Chem Mater 21:5079–5087. https://doi.org/10.1021/cm901618m
Yan A, Liu X, Qiu G et al (2008) Solvothermal synthesis and characterization of size-controlled Fe3O4 nanoparticles. J Alloys Compd 458:487–491. https://doi.org/10.1016/j.jallcom.2007.04.019
Wang Z, Yang W, Liu X (2014) Electrical properties of poly(arylene ether nitrile)/graphene nanocomposites prepared by in situ thermal reduction route. J Polym Res. https://doi.org/10.1007/s10965-014-0358-y
Ooi F, Duchene JS, Qiu J et al (2015) A facile solvothermal synthesis of octahedral Fe3O4 nanoparticles. Small 11:2649–2653. https://doi.org/10.1002/smll.201401954
Vetter J, Novák P, Wagner MR et al (2005) Ageing mechanisms in lithium-ion batteries. J Power Sources 147:269–281. https://doi.org/10.1016/j.jpowsour.2005.01.006
Okada S, Yamaki J (1989) Effect of particle size on iron-phthalocyanine cathodes in secondary lithium cells 136:2437–2440
Zhang W, Du L, Chen Z et al (2016) ZnO nanocrystals as anode electrodes for lithium-ion batteries. J Nanomater 2016:10–12. https://doi.org/10.1155/2016/8056302
Jiang Y, Jiang Z-J, Yang L et al (2015) A high-performance anode for lithium ion batteries: Fe3O4 microspheres encapsulated in hollow graphene shells. J Mater Chem A 3:11847–11856. https://doi.org/10.1039/C5TA01848J
Kim N, Oh C, Kim J et al (2017) High-performance Li-ion battery anodes based on silicon–graphene self-assemblies. J Electrochem Soc 164:A6075–A6083. https://doi.org/10.1149/2.0101701jes
Okada S, Yamaki J-I (1989) Intercalation mechanism in lithium/iron-phthalocyanine cells. J Electrochem Soc 136:340–344. https://doi.org/10.1149/1.2096631
Chen J, Guo J, Zhang T et al (2016) Electrochemical properties of carbonyl substituted phthalocyanines as electrode materials for lithium-ion batteries. RSC Adv 6:52850–52853. https://doi.org/10.1039/C6RA09826F
Acknowledgements
We greatly acknowledge the support of the Academic Support Program of University of Electronic Science and Technology of China (UESTC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10853_2018_2159_MOESM1_ESM.docx
Supplementary material 1 The synthetic route of the FePc, XRD and BET characterization of the materials, size distribution analysis of the FePc, equivalent circuit model for EIS measurements, TGA curves
Rights and permissions
About this article
Cite this article
He, D., Xue, W., Zhao, R. et al. Reduced graphene oxide/Fe-phthalocyanine nanosphere cathodes for lithium-ion batteries. J Mater Sci 53, 9170–9179 (2018). https://doi.org/10.1007/s10853-018-2159-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2159-x