Abstract
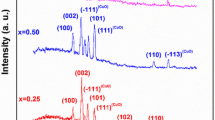
Y-zeolite was first modified by means of ions exchange with Al, Ca and Na, respectively. The modified materials were characterized by using X-ray diffraction (XRD), scanning electron microscopy (SEM) and inductively coupled plasma optical emission spectrometry (ICP-OES). Gas sensors were fabricated by SnO2 and coating Y-zeolites on the outside of SnO2 surface, respectively. It was found that the responses of the composites of all types of zeolite- and SnO2-based sensors became lower comparing with that of the pure SnO2-based one response of SnO2 sensor to ethanol vapor. It indicates a suppression effect of zeolites on the response to ethanol vapor. In contrast, the response of the composite-sensing materials of the modified Y-zeolite/SnO2-based sensors except the Ca-modified one to acetone indicates a significantly improved response, 2–3 times higher than that of pure SnO2-based sensor which is smaller than the one of SnO2 sensor. The possible mechanism of the effects of the Y-zeolites on the response of the sensors has been discussed.












Similar content being viewed by others
References
Judith Vijaya J, Kennedy LJ, Sekaran G, Bayhan M, William MA (2008) Preparation and VOC gas sensing properties of Sr(II)-added copper aluminate spinel composites. Sens Actuators B Chem 134(2):604–612. https://doi.org/10.1016/j.snb.2008.06.012
Kampa M, Castanas E (2008) Human health effects of air pollution. Environ Pollut 151(2):362–367. https://doi.org/10.1016/j.envpol.2007.06.012
Tokumura M, Nakajima R, Znad HT, Kawase Y (2008) Chemical absorption process for degradation of VOC gas using heterogeneous gas–liquid photocatalytic oxidation: toluene degradation by photo-Fenton reaction. Chemosphere 73(5):768–775. https://doi.org/10.1016/j.chemosphere.2008.06.021
Lee J-H (2009) Gas sensors using hierarchical and hollow oxide nanostructures: overview. Sens Actuators B Chem 140(1):319–336. https://doi.org/10.1016/j.snb.2009.04.026
Kim Y-S, Hwang I-S, Kim S-J, Lee C-Y, Lee J-H (2008) CuO nanowire gas sensors for air quality control in automotive cabin. Sens Actuators B Chem 135(1):298–303. https://doi.org/10.1016/j.snb.2008.08.026
Young-Jin C, In-Sung H, Jae-Gwan P, Kyoung Jin C, Jae-Hwan P, Jong-Heun L (2008) Novel fabrication of an SnO2 nanowire gas sensor with high sensitivity. Nanotechnology 19(9):095508. https://doi.org/10.1088/0957-4484/19/9/095508
Kim H-R, Choi K-I, Lee J-H, Akbar SA (2009) Highly sensitive and ultra-fast responding gas sensors using self-assembled hierarchical SnO2 spheres. Sens Actuators B Chem 136(1):138–143. https://doi.org/10.1016/j.snb.2008.11.016
Srivastava AK (2003) Detection of volatile organic compounds (VOCs) using SnO2 gas-sensor array and artificial neural network. Sens Actuators B Chem 96(1–2):24–37. https://doi.org/10.1016/S0925-4005(03)00477-5
Leite ER, Weber IT, Longo E, Varela JA (2000) A new method to control particle size and particle size distribution of SnO2 nanoparticles for gas sensor applications. Adv Mater 12(13):965–968. https://doi.org/10.1002/1521-4095(200006)12:13<965::aid-adma965>3.0.co;2-7
Lim C-B, Oh S (1996) Microstructure evolution and gas sensitivities of Pd-doped SnO2-based sensor prepared by three different catalyst-addition processes. Sens Actuators B Chem 30(3):223–231. https://doi.org/10.1016/0925-4005(96)80053-0
Duta A, Visa M (2015) Simultaneous removal of two industrial dyes by adsorption and photocatalysis on a fly-ash–TiO2 composite. J Photochem Photobiol A 306:21–30. https://doi.org/10.1016/j.jphotochem.2015.03.007
Reiß S, Schönauer D, Hagen G, Fischerauer G, Moos R (2011) Monitoring the ammonia loading of zeolite-based ammonia SCR catalysts by a microwave method. Chem Eng Technol 34(5):791–796. https://doi.org/10.1002/ceat.201000546
Shah R, Payne MC, Lee MH, Gale JD (1996) Understanding the catalytic behavior of zeolites: a first-principles study of the adsorption of methanol. Science 271(5254):1395–1397. https://doi.org/10.1126/science.271.5254.1395
Visa M, Andronic L, Duta A (2015) Fly ash–TiO2 nanocomposite material for multi-pollutants wastewater treatment. J Environ Manage 150:336–343. https://doi.org/10.1016/j.jenvman.2014.10.026
Yilmaz B, Müller U (2009) Catalytic applications of zeolites in chemical industry. Top Catal 52(6):888–895. https://doi.org/10.1007/s11244-009-9226-0
Güntner AT, Abegg S, Wegner K, Pratsinis SE (2018) Zeolite membranes for highly selective formaldehyde sensors. Sens Actuators B Chem 257(Supplement C):916–923. https://doi.org/10.1016/j.snb.2017.11.035
Caro J, Noack M (2008) Zeolite membranes—recent developments and progress. Microporous Mesoporous Mater 115(3):215–233. https://doi.org/10.1016/j.micromeso.2008.03.008
Jadsadapattarakul D, Thanachayanont C, Nukeaw J, Sooknoi T (2010) Improved selectivity, response time and recovery time by [0 1 0] highly preferred-orientation silicalite-1 layer coated on SnO2 thin film sensor for selective ethylene gas detection. Sens Actuators B Chem 144(1):73–80. https://doi.org/10.1016/j.snb.2009.10.035
Lai Z, Bonilla G, Diaz I, Nery JG, Sujaoti K, Amat MA, Kokkoli E, Terasaki O, Thompson RW, Tsapatsis M, Vlachos DG (2003) Microstructural optimization of a zeolite membrane for organic vapor separation. Science 300(5618):456–460. https://doi.org/10.1126/science.1082169
Li X, Dutta PK (2010) Interaction of dimethylmethylphosphonate with zeolite Y: impedance-based sensor for detecting nerve agent simulants. J Phys Chem C 114(17):7986–7994. https://doi.org/10.1021/jp100088w
Lin YS (2001) Microporous and dense inorganic membranes: current status and prospective. Sep Purif Technol 25(1–3):39–55. https://doi.org/10.1016/S1383-5866(01)00089-2
Varsani P, Afonja A, Williams DE, Parkin IP, Binions R (2011) Zeolite-modified WO3 gas sensors—enhanced detection of NO2. Sens Actuators B Chem 160(1):475–482. https://doi.org/10.1016/j.snb.2011.08.014
Vilaseca M, Coronas J, Cirera A, Cornet A, Morante JR, Santamaria J (2007) Gas detection with SnO2 sensors modified by zeolite films. Sens Actuators B Chem 124(1):99–110. https://doi.org/10.1016/j.snb.2006.12.009
Yang J-C, Dutta PK (2009) Solution-based synthesis of efficient WO3 sensing electrodes for high temperature potentiometric NOx sensors. Sens Actuators B Chem 136(2):523–529. https://doi.org/10.1016/j.snb.2008.09.017
Tarttelin Hernández P, Hailes SMV, Parkin IP (2017) Hydrocarbon detection with metal oxide semiconducting gas sensors modified by overlayer or admixture of zeolites Na-A, H-Y and H-ZSM-5. Sens Actuators B Chem 242:1281–1295. https://doi.org/10.1016/j.snb.2016.09.006
Vilaseca M, Coronas J, Cirera A, Cornet A, Morante JR, Santamaria J (2008) Development and application of micromachined Pd/SnO2 gas sensors with zeolite coatings. Sens Actuators B Chem 133(2):435–441. https://doi.org/10.1016/j.snb.2008.03.002
Huang H, Zhou J, Chen S, Zeng L, Huang Y (2004) A highly sensitive QCM sensor coated with Ag+-ZSM-5 film for medical diagnosis. Sens Actuators B Chem 101(3):316–321. https://doi.org/10.1016/j.snb.2004.04.001
Moos R, Müller R, Plog C, Knezevic A, Leye H, Irion E, Braun T, Marquardt K-J, Binder K (2002) Selective ammonia exhaust gas sensor for automotive applications. Sens Actuators B Chem 83(1–3):181–189. https://doi.org/10.1016/S0925-4005(01)01038-3
Aoki K, Tuan VA, Falconer JL, Noble RD (2000) Gas permeation properties of ion-exchanged ZSM-5 zeolite membranes. Microporous Mesoporous Mater 39(3):485–492. https://doi.org/10.1016/S1387-1811(00)00224-9
Hasegawa Y, Watanabe K, Kusakabe K, Morooka S (2001) The separation of CO2 using Y-type zeolite membranes ion-exchanged with alkali metal cations. Sep Purif Technol 22–23:319–325. https://doi.org/10.1016/S1383-5866(00)00154-4
Mann DP, Paraskeva T, Pratt KFE, Parkin IP, Williams DE (2005) Metal oxide semiconductor gas sensors utilizing a Cr-zeolite catalytic layer for improved selectivity. Meas Sci Technol 16(5):1193–1200
Xu K, Yuan C, Caro J, Huang A (2016) Silver-exchanged zeolite LTA molecular sieving membranes with enhanced hydrogen selectivity. J Membr Sci 511:1–8. https://doi.org/10.1016/j.memsci.2016.03.036
Lisnawati E, Krisnandi YK, Triyono D, Aip (2017) Modification of hybrid NaY/ZSM-5/IDC zeolite composite with exchanged Cu2+ and its application as ammonia gas sensor. In: International conference on chemistry, chemical process and engineering, vol 1823. AIP Conference Proceedings. https://doi.org/10.1063/1.4978169
Ward JW (1967) The nature of active sites on zeolites. J Catal 9(3):225–236. https://doi.org/10.1016/0021-9517(67)90248-5
Zheng Y, Li X, Dutta PK (2012) Exploitation of unique properties of zeolites in the development of gas sensors. Sensors 12(4):5170–5194
Shao Q, Huang LL, Zhou J, Lu LH, Zhang LZ, Lu XH, Jiang SY, Gubbins KE, Zhu YD, Shen WF (2007) Molecular dynamics study on diameter effect in structure of ethanol molecules confined in single-walled carbon nanotubes. J Phys Chem C 111(43):15677–15685. https://doi.org/10.1021/jp0736140
Inaba M, Murata K, Saito M, Takahara I (2006) Ethanol conversion to aromatic hydrocarbons over several zeolite catalysts. React Kinet Catal Lett 88(1):135–141. https://doi.org/10.1007/s11144-006-0120-5
Hathaway PE, Davis ME (1989) Base catalysis by alkali modified zeolites. J Catal 119(2):497–507. https://doi.org/10.1016/0021-9517(89)90177-2
Chen Y, Zhang Y, Li D, Gao F, Feng C, Wen S, Ruan S (2015) Humidity sensor based on AlPO4-5 zeolite with high responsivity and its sensing mechanism. Sens Actuators B Chem 212:242–247. https://doi.org/10.1016/j.snb.2015.02.017
Masuda T, Fujikata Y, Nishida T, Hashimoto K (1998) The influence of acid sites on intracrystalline diffusivities within MFI-type zeolites. Microporous Mesoporous Mater 23(3–4):157–167. https://doi.org/10.1016/S1387-1811(98)00058-4
Acknowledgements
The authors thank the National Natural Science Foundation of China (61574025, 61474012 and 51602035) for financial support. The authors also thank Mr. Liu Xuanzhou for his help in the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, Q., Wang, J., Sun, Y. et al. Gas-sensing properties of composites of Y-zeolite and SnO2. J Mater Sci 53, 6729–6740 (2018). https://doi.org/10.1007/s10853-018-2016-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2016-y