Abstract
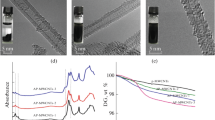
In this study, high-energy electron beam irradiation was used for the first time for graft polymerization of acrylic acid onto the surface of carbon black (CB) to prepare water-dispersible CB. The grafted CB was characterized by Fourier transform infrared spectroscopy, thermo-gravimetric analysis and X-ray photoelectron spectroscopy. The results indicate that polyacrylic acid (PAA) has been successfully grafted onto the surface of CB. The particle size and dispersion stability of unmodified and modified CBs in aqueous solution were determined by dynamic light scattering, transmission electron microscopy and ultraviolet–visible spectrophotometer. The results show that the grafted CB has smaller average aggregate size and better dispersion than unmodified CB. In addition, there is no significant difference in the grafting degree among grafted CBs prepared in nitrogen and air at different irradiation doses, indicating that oxygen and irradiation dose have a negligible effect on the grafting degree of PAA.











Similar content being viewed by others
References
Donnet JB, Bansal RC, Wang MJ (1993) Carbon black: science and technology, 2nd edn. Marcel Dekker, New York. ISBN 082478975X
West DHD, Mcbrierty VJ, Delaney CFG (1979) A positron annihilation study of carbon black and carbon-black-filled polybutadiene. Appl Phys 18:85–92. https://doi.org/10.1007/s10853-017-1680-7
Iijima M, Yamazaki M, Nomura Y, Kamiya H (2013) Effect of structure of cationic dispersants on stability of carbon black nanoparticles and further processability through layer-by-layer surface modification. Chem Eng Sci 85:30–37. https://doi.org/10.1016/j.ces.2012.02.020
Zhang W, Blackburn RS, DehghaniSanij AA (2009) Carbon black reinforced epoxy resin nanocomposites as bending sensors. J Compos Mater 43:367–376. https://doi.org/10.1177/0021998308099308
Francis LF, Grunlan JC, Sun J, Gerberich WW (2007) Conductive coatings and composites from latex-based dispersions. Colloids Surf A 311:48–54. https://doi.org/10.1016/j.colsurfa.2007.08.026
Zhu L, Lu Y, Wang Y, Zhang L, Wang W (2012) Preparation and characterization of dopamine-decorated hydrophilic carbon black. Appl Surf Sci 258:5387–5393. https://doi.org/10.1016/j.apsusc.2012.02.016
Zhou X, Li Q, Wu C (2008) Grafting of maleic anhydride onto carbon black surface via ultrasonic irradiation. Appl Organomet Chem 22:78–81. https://doi.org/10.1002/aoc.1352
Hauptman N, Gunde MK, Kunaver M, Bešter-Rogac M (2011) Influence of dispersing additives on the conductivity of carbon black pigment dispersion. J Coat Technol Res 8:553–561. https://doi.org/10.1007/s11998-011-9330-5
Shi PW, Li QY, Li YC, Wu CF (2014) Preparation and characterization of poly(sodium 4-styrenesulfonate)-decorated hydrophilic carbon blackby one-step in situ ball milling. Colloids Surf A 443:135–140. https://doi.org/10.1016/j.colsurfa.2013.10.060
Buttrya DA, Pengb JCM, Donnetb JB, Rebouillatc S (1999) Immobilization of amines at carbon fiber surfaces. Carbon 37:1929–1940. https://doi.org/10.1016/s0008-6223(99)00064-0
Donnet JB, Rebouillat S, Wang TK, Peng J (1998) Carbon fibers, 3rd edn. Marcel Dekker Inc, New York. ISBN 0824701720
Chen X, Farber M, Gao Y, Kulaots I et al (2003) Mechanisms of surfactant adsorption on non-polar, air-oxidized and ozone-treated carbon surfaces. Carbon 41:1489–1500. https://doi.org/10.1016/s0008-6223(03)00053-8
Li Q, Wu G, Ma Y, Wu C (2007) Grafting modification of carbon black by trapping macroradicals formed by sonochemical degradation. Carbon 45:2411–2416. https://doi.org/10.1016/j.carbon.2007.06.052
Itoh Y, Ozaki K, Maezawa R (2013) Hydrolyzable-emulsifier-containing polymer latices as dispersants and binders for waterborne carbon black paint. J Appl Polym Sci 130:3869–3873. https://doi.org/10.1002/app.39479
Tsubokawa N, Satoh T, Murota M, Sato S, Shimizu H (2001) Grafting of hyperbranched poly(amidoamine) onto carbon black surfaces using dendrimer synthesis methodology. Polym Adv Technol 12:596–602. https://doi.org/10.1002/pat.148
Liu T, Jia S, Tomasz Kowalewski A, Matyjaszewski K et al (2003) Grafting poly(n-butyl acrylate) from a functionalized carbon black surface by atom transfer radical polymerization. Langmuir 19:6342–6345. https://doi.org/10.1021/la034219d
Reichmanis E, Nalamasu O, Houlihan FM, Novembre AE (2015) Radiation chemistry of polymeric materials: novel chemistry and applications for microlithography. Polym Int 48:1053–1059. https://doi.org/10.1002/(SICI)1097-0126(199910)48:10<1053::AID-PI268>3.0.CO;2-T
Iwata H, Nakanoya T, Morohashi H, Chen J et al (2006) Novel gas and contamination sensor materials from polyamide-block-poly(ethylene oxide)-grafted carbon black. Sens Actuat B Chem 113:875–882. https://doi.org/10.1109/icsens.2003.1279080
Singh D, Singh NL, Qureshi A, Gavade C, Avasthi DK et al (2010) Electrical and thermal studies on the polyvinylchloride/carbon black composites induced by high energy ion beam. Integr Ferroelectr 117:85–96. https://doi.org/10.1080/10584587.2010.489429
Trenikhin MV, Ivashchenko OV, Eliseev VS (2015) Electron microscopy investigation of structural transformation of carbon black under influence of high-energy electron beam. Fuller Nanotub Carbon Nanostruct 23:801–806. https://doi.org/10.1080/1536383x.2014.1003639
Sapinski M, Dehning B, Guerrero A, Meyer M, Kroyer T, Switzerland G, Carbon fiber damage in particle beam. In: Proceedings of HB2010, Morschach, Switzerland
Wu Y, Wen S, Shen J, Jiang J, Hu S, Zha L, Liu L (2015) Improved dynamic properties of natural rubber filled with irradiation-modified carbon black. Radiat Phys Chem 111:91–97. https://doi.org/10.1016/j.radphyschem.2015.02.020
Sahoo BP, Naskar K, Dubey KA, Choudhary RNP, Tripathy DK (2013) Study of dielectric relaxation behavior of electron beam-cured conductive carbon black-filled ethylene acrylic elastomer. J Mater Sci 48:702–713. https://doi.org/10.1007/s10853-012-6782-7
Ahmad A, Mohd DH, Abdullah I (2004) Electron beam irradiation of carbon black filled linear low-density polyethylene. J Mater Sci 39:1459–1461. https://doi.org/10.1023/b:jmsc.0000013917.04266.79
Zhou X, Li Y, Fang C, Li S, Cheng Y, Lei W, Meng X (2015) Recent advances in synthesis of waterborne polyurethane and their application in water-based ink: a review. J Mater Sci Technol 31:708–722. https://doi.org/10.1016/j.jmst.2015.03.002
Bo Y, Cui J, Cai Y, Xu S (2016) Preparation and characterization of poly(methylmethacrylate) and poly(maleicanhydride-co-diallylphthalate) grafted carbon black through γ-ray irradiation. Radiat Phys Chem 119:236–246. https://doi.org/10.1016/j.radphyschem.2015.11.005
Xu H, Cao Y, He X, Wu Y, Zhang Y, Wu C (2009) Influence of in situ grafting on the dispersion of carbon black in solvents and natural rubber. J Macromol Sci B 48:1190–1200. https://doi.org/10.1080/00222340903275768
Socrates G (1994) Infrared characteristic group frequencies: tables and charts, 2nd edn. Wiley, New York. ISBN 0471852988
Lee S, Lee H, Sim JH, Sohn D (2014) Graphene oxide/poly(acrylic acid) hydrogel by γ-ray pre-irradiation on graphene oxide surface. Macromol Res 22:165–172. https://doi.org/10.1007/s13233-014-2025-x
Ding W, Wang L (2014) Synthesis of poly(acrylic acid) grafted carbon black and its application for sensing ethanol. J Polym Res 21:1–7. https://doi.org/10.1007/s10965-014-0425-4
Li Q, Wu G, Zhang X, Wu C (2006) Preparation of poly(n-butyl acrylates) en-capsulated carbon black via ultrasonic irradiation initiating emulsion poly-merization. Polym J 38:1245–1250. https://doi.org/10.1295/polymj.pj2006053
Lu S, Duan M, Lin S (2003) Synthesis of superabsorbent starch-graft-poly(potassiumacrylate-co-acrylamide) and its properties. J Appl Polym Sci 88:1536–1542. https://doi.org/10.1002/app.12025
Zhu L, Zhang L, Tang Y, Yang J (2013) Synthesis and adsorption of organo-montmorillonite/poly(acrylic acid) superabsorbent composite. Polym Polym Compos 21:21–26. https://doi.org/10.1016/j.polymertesting.2013.01.001
Ding W, Wang L, Yang Q et al (2013) Recent research progress on polymer grafted carbon black and its novel applications. Int Polym Proc 28:132–142. https://doi.org/10.3139/217.2678
Strzemiecka B, Voelkel A, Donate-Robles J, Martín-Martínez JM (2014) Assessment of the surface chemistry of carbon blacks by TGA-MS, XPS and inverse gas chromatography using statistical chemometric analysis. Appl Surf Sci 316:315–323. https://doi.org/10.1016/j.apsusc.2014.07.174
Liu H, Wang S, Xiao Y, Li X (2016) Studies on the dispersity of polymethacrylate-grafted carbon black in a non-aqueous medium: the influence of monomer structure. J Mater Sci-Mater Electron 27:2022–2030. https://doi.org/10.1007/s10854-015-3986-z
Bao Y, Huang J, Xue P, Wang J, Li Q, Wu C, Guo W (2011) Effect of pH-responsive on the dispersion of PVM/MA grafted carbon black in water and waterborne polyurethane. J Dispers Sci Technol 32:1459–1464. https://doi.org/10.1080/01932691.2010.513312
Acknowledgements
This research is financially supported by the Thousand Talents Program of Qinghai Province and Kunlun Scholar Award Program of Qinghai Province.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, Q., Wang, S. & Xu, S. Preparation and characterization of water-dispersible carbon black grafted with polyacrylic acid by high-energy electron beam irradiation. J Mater Sci 53, 6106–6115 (2018). https://doi.org/10.1007/s10853-017-1966-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1966-9