Abstract
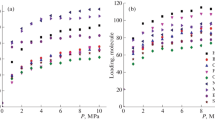
Grand canonical Monte Carlo method (GCMC) simulation combined with density functional theory calculation was used to investigate the adsorption in Cu-BTC, with nitrogen- and lithium-doping functionalization in order to understand the underlying performance of MOFs in methanol adsorption. A new N-doping structure, 3N–CuBTC, was theoretically constructed by replacing the carbon atoms with hydrogen connection in the organic linkers by nitrogen atoms. 3N–CuBTC shows higher methanol capacity in the measured pressure range due to the increased dispersive interactions caused by the lone electron pair of nitrogen atoms. Another Li-doping structure, 3Li–3N–CuBTC, was fabricated by doping Li atoms on the base of 3N–CuBTC. 3Li–3N–CuBTC demonstrated higher methanol capacity due to the stronger interaction between the induced Li atoms and methanol molecules. Furthermore, these two results can be attributed to the new adsorption sites created by N- and Li-doping, as revealed by the more exothermic binding energies on N-sites (− 49.37 kJ mol−1) and Li-sites (− 122.28 kJ mol−1) than Cu-sites (− 40.62 kJ mol−1). According to the simulation results, it can be concluded that both functionalized Cu-BTCs are capable of enhancing the methanol adsorption capacity of the framework at pressure from 0 to 14 kPa at 298 K.








Similar content being viewed by others
References
Khan NA, Hasan Z, Jhung SH (2013) Adsorptive removal of hazardous materials using metal–organic frameworks (MOFs): a review. J Hazard Mater 244:444–456
Dhakshinamoorthy A, Garcia H (2012) Catalysis by metal nanoparticles embedded on metal–organic frameworks. Chem Soc Rev 41:5262–5284
Zheng HQ, Zhang YN, Liu LF, Wan W, Guo P, Andreas MN, Zou XD (2016) One-pot synthesis of metal–organic frameworks with encapsulated target molecules and their applications for controlled drug delivery. J Am Chem Soc 138:962–968
Park J, Kim H, Jung YS (2013) Origin of selective guest-induced magnetism transition in Fe/MOF-74. J Phys Chem Lett 4:2530–2534
Gole B, Bar AK, Mukherjee PS (2011) Fluorescent metal–organic framework for selective sensing of nitroaromatic explosives. Chem Commun 47:12137–12139
Xu H, He YB, Zhang ZJ, Xiang SC, Cai JF, Cui YJ, Yang Y, Qian GD, Chen BL (2013) A microporous metal–organic framework with both open metal and Lewis basic pyridyl sites for high C2H2 and CH4 storage at room temperature. Chem Commun 49:6719–6721
Li YW, Li JR, Wang LF, Zhou BY, Chen Q, Bu XH (2013) Microporous metal–organic frameworks with open metal sites as sorbents for selective gas adsorption and fluorescence sensors for metal ions. J Mater Chem A 1:495–499
Zhang YM, Li BY, Krishna R, Wu ZL, Ma DX, Shi Z, Pham T, Forrest K, Space B, Ma SQ (2015) Highly selective adsorption of ethylene over ethane in a MOF Featuring the combination of open metal site and π-complexation. Chem Commun 51:2714–2717
Calero S, Gomez-Alvarez P (2015) Insights into the adsorption of water and small alcohols on the open-metal sites of Cu-BTC via molecular simulation. J Phys Chem C 119:467–472
Chui SSY, Lo SMF, Charmant JP, Orpen AG, Williams ID (1999) A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]N. Science 283:1148–1150
Du M, Li LN, Li MX, Si R (2016) Adsorption mechanism on metal organic frameworks of Cu-BTC, Fe-BTC and ZIF-8 for CO2 capture investigated by X-ray absorption fine structure. RSC Adv 6:62705–62716
Wang H, Qu ZG, Zhang W, Yu QN, He YL (2016) Experimental and numerical study of CO2 adsorption on copper benzene-1,3,5-tricarboxylate (Cu-BTC) metal organic framework. Int J Heat Mass Transf 92:859–863
Gutiérrez-Sevillano JJ, Calero S, Krishna R (2015) Selective adsorption of water from mixtures with 1-alcohols by exploitation of molecular packing effects in CuBTC. J Phys Chem C 119:3658–3666
Sezginel KB, Keskin S, Uzun A (2016) Tuning the gas separation performance of CuBTC by ionic liquid incorporation. Langmuir 32:1139–1147
Grajciar L, Blusky O, Nachtigall P (2010) Water adsorption on coordinatively unsaturated sites in CuBTC MOF. J Phys Chem Lett 1:3354–3359
Wang Q, Wang H, Peng SM, Peng X, Cao DP (2014) Adsorption and separation of Xe in metal–organic frameworks and covalent–organic materials. J Phys Chem C 118:10221–10229
Perry JJ, Teich-McGoldrick SL, Meek ST, Greathouse JA, Haranczyk M, Allendorf MD (2014) Noble gas adsorption in metal–organic frameworks containing open metal sites. J Phys Chem C 118:11685–11698
Silva B, Solomon I, Ribeiro AM, Lee UH, Hwang YK, Chang JS, Loureiro JM, Rodrigues AE (2013) H2 purification by pressure swing adsorption using CuBTC. Sep Purif Technol 118:744–756
Lin KS, Adhikari AK, Ku CN, Chiang CL, Kuo H (2012) Synthesis and characterization of porous HKUST-1 metal organic frameworks for hydrogen storage. Int J Hydrogen Energy 37:13865–13871
Chowdhury P, Mekala S, Dreisbach F, Gumma S (2012) Adsorption of CO, CO2 and CH4 on Cu-BTC and MIL-101 metal organic frameworks: effect of open metal sites and adsorbate polarity. Microporous Mesoporous Mater 152:246–252
Wu D, Guo X, Sun H, Navrotsky A (2015) Thermodynamics of methane adsorption on copper HKUST-1 at low pressure. J Phys Chem Lett 6:2439–2443
Oertel K, Fischer M (1998) Adsorption cooling for cold storage using methanol/silicagel. Appl Therm Eng 18:773–786
Schicktanz M, Hügenell P, Henninger SK (2012) Evaluation of methanol/activated carbons for thermally driven chillers, part II: the energy balance model. Int J Refrig 35:554–561
Henninger SK, Schicktanz M, Hügenell PPC, Sievers H, Henning H-M (2012) Evaluation of methanol adsorption on activated carbons for thermally driven chillers part I: thermophysical characterisation. Int J Refrig 35:543–553
Restuccia G, Freni A, Russo F, Vasta S (2005) Experimental investigation of a solid adsorption chiller based on a heat exchanger coated with hydrophobic zeolite. Appl Therm Eng 25:1419–1428
Jeremias F, Fröhlich D, Janiak C, Henninger SK (2014) Water and methanol adsorption on MOFs for fast cycling heat transformation processes, new. J Chem 38:1846–1852
Rezk A, AL-Dadah R, Mahmoud S, Elsayed A (2013) Investigation of ethanol/metal organic frameworks for low temperature adsorption cooling applications. Appl Energy 112:1025–1031
Sha YF, Bai SZ, Lou J, Wu D, Liu BZ, Ling Y (2016) Tuning the adsorption behaviors of water, methanol, and ethanol in a porous material by varying the flexibility of substituted groups. Dalton Trans 45:7235–7239
Kummer H, Baumgartner M, Hügenell P, Fröhlich D, Henninger SK, Gläser R (2017) Thermally driven refrigeration by methanol adsorption on coatings of HKUST-1 and MIL-101(Cr). Appl Therm Eng 117:689–697
Sachdeva S, Venkatesh MR, Mansouri BE, Wei J, Bossche A, Kapteijn F, Zhang GQ, Gascon J, Smet LCPM, Sudholter EJR (2017) Sensitive and reversible detection of methanol and water vapor by in situ electrochemically grown CuBTC MOFs on interdigitated electrodes. Small 13:1064150–1064155
Nguyen BT, Nguyen HL, Nguyen TC, Cordova KE, Furukawa H (2016) High methanol uptake capacity in two new series of metal–organic frameworks: promising materials for adsorption-driven heat pump applications. Chem Mater 28:6243–6249
Ernst SJ, Jeremias F, Bart HJ, Henninger SK (2016) Methanol adsorption on HKUST-1 coatings obtained by thermal gradient deposition. Ind Eng Chem Res 55:13094–13101
Van Assche TRC, Duerinck T, Gutiérrez Sevillano JJ, Calero S, Baron GV, Denayer JFM (2013) High adsorption capacities and two-step adsorption of polar adsorbates on copper-benzene-1,3,5-tricarboxylate metal–organic framework. J Phys Chem C 117:18100–18111
Gutiérrez-Sevillano JJ, Vincent-Luna JM, Dubbeldam D, Calero S (2013) Molecular mechanisms for adsorption in Cu-BTC metal organic framework. J Phys Chem C 117:11357–11366
Yu C, Bourrelly S, Martineau C, Saidi F, Bloch E, Lavrard H, Taulelle F, Horcajada P, Serre C, Llewellyn PL, Magnier E, Devic T (2015) Functionalization of Zr-Based MOFs with alkyl and perfluoroalkyl groups: the effect on the water sorption behavior. Dalton Trans 44:19687–19692
Huang HL, Zhang WJ, Yang F, Wang B, Yang QY, Xie YB, Zhong CL, Li JR (2016) Enhancing CO2 adsorption and separation ability of Zr(IV)-based metal–organic frameworks through ligand functionalization under the guidance of the quantitative structure–property relationship. Model Chem Eng J 289:247–253
Debatin F, Thomas A, Kelling A, Hedin N, Bacsik Z, Senkovska I, Kaskel S, Junginger M, Müller H, Schilde U, Jäger C, Friedrich A, Holdt HJ (2010) In situ synthesis of an imidazolate-4-amide-5-imidate ligand and formation of a microporous zinc–organic framework with H2 and CO2-storage ability. Angew Chem Int Ed 49:1258–1262
Banerjee R, Phan A, Wang B, Knobler C, Furukawa H, O’Keeffe M, Yaghi OM (2008) High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 319:939–943
Liu K, Li BY, Li Y, Li X, Yang F, Zeng G, Peng Y, Zhang ZJ, Li GH, Shi Z, Feng SH, Song DT (2014) An N-rich metal–organic framework with an rht topology: high CO2 and C2 hydrocarbons uptake and selective capture from CH4. Chem Commun 50:5031–5033
Wu Y, Liu DF, Chen HY, Qian Y, Xi HX, Xia QB (2015) Enhancement effect of lithium-doping functionalization on methanol adsorption in copper-based metal–organic framework. Chem Eng Sci 123:1–10
Srinivasu K, Ghosh SK (2011) Tuning the metal binding energy and hydrogen storage in alkali metal decorated MOF-5 through boron doping: a theoretical investigation. J Phys Chem C 115:16984–16991
Sun D, Ma SQ, Ke YX, Petersen TM, Zhou HC (2005) Synthesis, characterization, and photoluminescence of isostructural Mn Co, and Zn MOFs having a diamondoid structure with large tetrahedral cages and high thermal stability. Chem Commun 3:2663–2665
Han SS, Goddard WA (2007) Lithium-doped metal–organic frameworks for reversible H2 storage at ambient temperature. J Am Chem Soc 219:8422–8423
Dassault Systèmes BIOVIA (2017) Materials Studio 2017. Dassault Systèmes, San Diego
Xiang ZH, Hu Z, Cao DP, Yang QT, Lu JM, Han BY, Wang WC (2011) Metal-organic frameworks with incorporated carbon nanotubes: improving carbon dioxide and methane storage capacities by lithium doping. Angew Chem Int Ed 50:491–494
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE (2009) Gaussian 09, revision A. 02, vol 19. Gaussian, Inc, Wallingford, pp 227–238
Yang QY, Zhong CL (2007) Molecular simulation of carbon dioxide/methane/hydrogen mixture adsorption in metal–organic frameworks. J Phys Chem B 110:17776–17783
Jorgensen WL, Maxwell DS, Rives JT (1996) Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J Am Chem Soc 118:11225–11236
Rappé AK, Casewit CJ, Colwell KS, Goddard WA III, Skid WM (1992) UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J Am Chem Soc 114:10024–10035
Chen B, Potoff JJ, Siepmann JI (2001) Monte Carlo calculations for alcohols and their mixtures with alkanes. transferable potentials for phase equilibria. 5. united-atom description of primary, secondary, and tertiary alcohols. J Phys Chem B 105:3093–3104
Gupta A, Chempath S, Sanborn MJ, Clark LA, Snurr RQ (2003) Object-oriented programming paradigms for molecular modeling. Mol Simul 29:29–46
Cho J, Rho S, Park SJ, Lee JC, Kim HY (1998) A comparison between Anderko’s model with composition-dependent physical interaction parameters and Srk-based Hexamer model for HFC and HF Systems. Fluid Phase Equilib 144:69–75
Düren T, Snurr RQ (2014) Assessment of isoreticular metal–organic frameworks for adsorption separations: a molecular simulation study of methane/n-butane mixtures. J Phys Chem B 108:15703–15708
Talu O, Myers AL (2001) Molecular simulation of adsorption: Gibbs dividing surface and comparison with experiment. AIChE J 47:1160–1168
Sarkisov L, Harrison A (2011) Computational structure characterisation tools in application to ordered and disordered porous materials. Mol Simul 37:1248–1257
Mavrandonakis A, Tylianakis E, Stubos AK, Froudakis GE (2008) Why Li doping in MOFs enhances H2 storage capacity? A multi-scale theoretical study. J Phys Chem C 112:7290–7294
Liu Y, Liu J, Lin YS (2015) Strong binding site molarity of MOFs and its effect on CO2 adsorption. Microporous Mesoporous Mater 214:242–245
Wen HM, Li B, Wang HL, Krishna R, Chen BL (2016) High acetylene/ethylene separation in a microporous zinc(II) metal–organic framework with low binding energy. Chem Commun 52:1166–1169
Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (Nos. 21436005 and 21576094), the National High Technology Research and Development Program of China (No. 2013AA065005), SRFDP (No. 20130172110012).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Z., Zhang, K., Wu, Y. et al. Effective enhancement on methanol adsorption in Cu-BTC by combination of lithium-doping and nitrogen-doping functionalization. J Mater Sci 53, 6080–6093 (2018). https://doi.org/10.1007/s10853-017-1751-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1751-9