Abstract
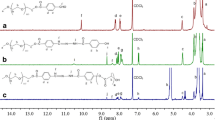
A new type of acid-sensitive 100% hyperbranched polyacetals (HBPA) was synthesized, which could be completely degraded into small molecules under acidic environment and avoid the accumulative toxicity in vivo. The AB2 monomer was synthesized by 4-carboxybenzaldehyde and 2-bromoethanol. The bulk polycondensation was carried out in vacuum environment to remove water byproduct. The massive terminal aldehyde groups of HBPA were conjugated with mPEG-NH2 and doxorubicins to form amphiphilic acid-sensitive polymer–drug conjugates (DOX-HBPA-PEG). The stability of the micelles of DOX-HBPA-PEG was evaluated by DLS at different pH value in phosphate buffer saline (PBS). The DOX release in vitro showed that the cumulative release rate was 14.51% in pH 7.4 PBS after 24 h and the cumulative release rate was 48.56% in pH 6.0 PBS after 24 h. The results of cell viability of DOX-HBPA-PEG and HBPA-PEG showed that the polymer–DOX conjugates were effective drug delivery systems. The uptake process of DOX-HBPA-PEG by A549 cells showed that the micelle was totally swallowed in 1 h later. The controllable drug release nature, stability, biocompatibility and completely degradable structures (acid-sensitive) make them to be promising drug delivery systems.











Similar content being viewed by others
References
Zhang P, Cheng F, Zhou R, Cao J, Li J, Burda C, Min Q, Zhu J (2014) DNA-hybrid-gated multifunctional mesoporous silica nanocarriers for dual-targeted and microRNA-responsive controlled drug delivery. Angew Chem Int Ed 126:2403–2407
Ma M, Huang Y, Chen H, Jia X, Wang S, Wang Z, Shi J (2015) Bi2S3-embedded mesoporous silica nanoparticles for efficient drug delivery and interstitial radiotherapy sensitization. Biomaterials 37:447–455
Geers B, Dewitte H, De Smedt SC, Lentacker I (2012) Crucial factors and emerging concepts in ultrasound-triggered drug delivery. J Control Release 164:248–255
Soldano C (2015) Hybrid metal-based carbon nanotubes: Novel platform for multifunctional applications. Prog Mater Sci 69:183–212
Chen P, Hsiao KM, Chou C (2013) Molecular characterization of toxicity mechanism of single-walled carbon nanotubes. Biomaterials 34:5661–5669
Fan L, Campagnoli S, Wu H, Grandi A, Parri M, Camilli ED, Grandi G, Viale G, PileriP Grifantini R, Song CJ, Jin B (2015) Negatively charged AuNP modified with monoclonal antibody against novel tumor antigen FAT1 for tumor targeting. J Exp Clin Cancer Res 34:103
Fan L, Zhang YS, Wang F, Yang Q, Tan J, Grifantini R, Wu H, Song CJ, Jin B (2016) Multifunctional all-in-one drug delivery systems for tumor targeting and sequential release of three different anti-tumor drugs. Biomaterials 76:399–407
Amiri A, Ramazani A, Jahanshahi M, Moghadamnia AA (2016) Synthesis and evaluating of nanoporous molecularly imprinted polymers for extraction of quercetin as a bioactive component of medicinal plants, Iran. J Chem Chem Eng 35(4):11–19
Malekzadeh AM, Ramazani A, Rezaei SJT, Niknejad H (2017) Design and construction of multifunctional hyperbranched polymers coated magnetite nanoparticles for both targeting magnetic resonance imaging and cancer therapy. J Colloid Interface Sci 490:64–73
Dayyani N, Khoee S, Ramazani A (2015) Design and synthesis of pH-sensitive polyamino-ester magnetodendrimers: surface functional groups effect on viability of human prostate carcinoma cell lines DU145. Eur J Med Chem 98:190–202
QiaoYB Duan X, Fan L, Li W, Wu H, Wang YK (2014) Synthesis of controlled molecular weight poly (β-malic acid) and conjugation with HCPT as a polymeric drug carrier. J Polym Res 21:397–405
Li F, Zhang HT, Gu CH, Fan L, QiaoYB Tao YC, Cheng C, Wu H, Yi J (2013) Self-assembled nanoparticles from folate-decorated maleilated pullulan–doxorubicin conjugate for improved drug delivery to cancer cells. Polym Int 62:165–171
Li F, Fan L, Li W, Duan X, QiaoYB WuH (2014) Synthesis and micellar characterization of luteinizing hormone-releasing hormone poly(ethylene glycol)- block-poly(l-histidine) copolymers. Polym Eng Sci 55(2):277–286
Fang J, Nakamura H, Maeda H (2011) The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 63:136–151
Tetsuji Y, Yasuhiko T, Yoshito I (1994) Distribution and tissue uptake of poly(ethylene glycol) with different molecular weights after intravenous administration to mice. J Pharm Sci-US 84:601–606
Tetsuji Y, Yasuhiko T, Yoshito I (1995) Fate of water-soluble polymers administered via different routes. J Pharm Sci-US 84:349–354
Martin EE, Sebastian S, Marc D (2004) Anti-vascular tumor therapy: recent advances, pitfalls and clinical perspectives. Drug Resist Update 7:125–138
Markovsky E, Baabur-Cohen H, Eldar-Boock A, Omer L, Tiram G, Ferber S, Ofek P, Polyak D, Scomparin A, Satchi-Fainaro R (2012) Administration, distribution, metabolism and elimination of polymer therapeutics. J Control Release 161:446–460
Hong BJ, Chipre AJ, Nguyen ST (2013) Acid-degradable polymer-caged lipoplex (PCL) platform for siRNA delivery: facile cellular triggered release of siRNA. J Am Chem Soc 135:17655–17658
Kumar A, Lale SV, Mahajan S, Choudhary V, Koul V (2015) ROP and ATRP fabricated dual targeted redox sensitive polymersomes based on pPEGMA-PCL-ss-PCL-pPEGMA triblock copolymers for breast cancer therapeutics. ACS Appl Mater Interfaces 7:9211–9227
Sitia L, Ferrari R, Violatto MB, Talamini L, Dragoni L et al (2016) Fate of PLA and PCL-based polymeric nanocarriers in cellular and animal models of triple-negative breast cancer. Biomacromol 17:744–755
Man DKW, Casettari L, Cespi M, Bonacucina G, Palmieri GF et al (2015) Oleanolic acid loaded PEGylated PLA and PLGA nanoparticles with enhanced cytotoxic activity against cancer cells. Mol Pharm 12:2112–2125
Nascimento AV, Singh A, Bousbaa H, Ferreira D, Sarmento B et al (2015) Combinatorial-designed epidermal growth factor receptor-targeted chitosan nanoparticles for encapsulation and delivery of lipid-modified platinum derivatives in wild-type and resistant non-small-cell lung cancer cells. Mol Pharm 12:4466–4477
Ferreira DP, Conceição DS, Fernandes F, Sousa T, Calhelha RC et al (2016) Characterization of a squaraine/chitosan system for photodynamic therapy of cancer. J Phys Chem B 120:1212–1220
Kiani M, MirzazadehTekie FS, Dinarvand M, Soleimani M, Dinarvand R et al (2016) Thiolatedcarboxymethyl dextran as a nanocarrier for colon delivery of hSET1 antisense: in vitro stability and efficiency study. Mater Sci Eng C-Mater 62:771–778
Anirudhan TS, Binusreejayan (2016) Dextran based nanosized carrier for the controlled and targeted delivery of curcumin to liver cancer cells. Int J Biol Macromol 88:222–235
Tarvirdipour S, Vasheghani-Farahani E, Soleimani M, Bardania H (2016) Functionalized magnetic dextran-spermine nanocarriers for targeted delivery of doxorubicin to breast cancer cells. Int J Pharm 501:331–341
Tomalova B, Sirova M, Rossmann P, Pola R, Strohalm J et al (2016) The structure-dependent toxicity, pharmacokinetics and anti-tumour activity of HPMA copolymer conjugates in the treatment of solid tumours and leukaemia. J Control Release 223:1–10
Mao J, Li Y, Wu T, Yuan C, Zeng B et al (2016) A simple dual-pH responsive prodrug-based polymeric micelles for drug delivery. ACS Appl Mater Interfaces 8:17109–17117
Zhang Y, Xiao C, Ding J, Li M, Chen X et al (2016) A comparative study of linear, Y-shaped and linear-dendritic methoxy poly(ethylene glycol)-block-polyamidoamine-block-poly(l-glutamic acid) block copolymers for doxorubicin delivery in vitro and in vivo. Acta Biomater 40:243–253
Chen P, Qiu M, Deng C, Meng F, Zhang J et al (2015) pH-responsive chimaericpepsomes based on asymmetric poly(ethyleneglycol)-b-poly(l-leucine)-b-poly(l-glutamic acid) triblock copolymer for efficient loading and active intracellular delivery of doxorubicin hydrochloride. Biomacromolecules 16:1322–1330
Chen FM, Lu H, Wu LA, Gao LN, An Y et al (2013) Surface-engineering of glycidylmethacrylated dextran/gelatin microcapsules with thermo-responsive poly(N-isopropylacrylamide) gates for controlled delivery of stromal cell-derived factor-1α. Biomaterials 34:6515–6527
Li F, Wu H, Fan L, Zhang HT, Zhang H, Gu CH (2009) Study of dual responsive poly[(maleilated dextran)-graft-(N-isopropylacrylamide)] hydrogel nanoparticles: preparation, characterization and biological evaluation. Polym Int 58:1023–1033
Fan L, Li F, Zhang HT, Wang YK, Cheng C, Li XY, Gu CH, Yang Q, Wu H, Zhang SY (2010) Co-delivery of PDTC and doxorubicin by multifunctional micellar nanoparticles. Biomaterials 31:5634–5642
Wu H, Zhu L, Torchilin VP (2013) pH-sensitive poly(histidine)-PEG/DSPE-PEG co-polymer micelles for cytosolic drug delivery to achieve active targeted drug delivery and overcome multidrug resistance. Biomaterials 34:1213–1222
Li DD, Wang JX, Ma Y, Qian HS, Wang D, Wang L, Zhang GB, QiuLZ Wang YC, Yang XZ (2016) A donor–acceptor conjugated polymer with alternating isoindigo derivative and bithiophene units for near-infrared modulated cancer thermo-chemotherapy. ACS Appl Mater Interfaces 8:19312–19320
Wang JX, Liu Y, Ma YC, Sun CY, Tao W, Wang YC, Yang XZ, Wang J (2016) NIR-activated supersensitive drug release using nanoparticles with a flow core. Adv Funct Mater 26:7516–7525
Zhang S, Chen H, Kong J (2016) Disulfide bonds-containing amphiphilic conetworks with tunable reductive-cleavage. RSC Adv 6:36568–36575
Duan X, Chen H, Fan L, Kong J (2016) Drug self-assembled delivery system with dual responsiveness for cancer chemotherapy. ACS Biomater Sci Eng 2:2347–2354
Dayyani N, Ramazani A, Khoee S, Shafiee A (2017) Synthesis and characterization of the first generation of polyamino-ester dendrimer-grafted magnetite nanoparticles from 3-aminopropyltriethoxysilane (APTES) via the convergent approach. Silicon 12:1–7
Tarasi R, Khoobi M, Niknejad H, Ramazani A, Ma’mani L, Bahadorikhalili S, Shafiee A (2016) β-Cyclodextrin functionalized poly(5-amidoisophthalicacid) grafted Fe3O4 magnetic nanoparticles: a novel biocompatible nanocomposite for targeted docetaxel delivery. J Magn Magn Mater 417:451–459
An X, Zhu A, Luo H, Ke H, Chen H et al (2016) Rational design of multi-stimuli-responsive nanoparticles for precise cancer therapy. ACS Nano 10:5947–5958
Talelli M, Vicent MJ (2014) Reduction sensitive poly(l-glutamic acid) (PGA)-protein conjugates designed for polymer masked-unmasked protein therapy. Biomacromolecules 15:4168–4177
Liu N, Vignolle J, Vincent J-M, Robert F, Landais Y et al (2014) One-pot synthesis and PEGylation of hyperbranched polyacetals with a degree of branching of 100%. Macromolecules 47:1532–1542
Parmar RG, Busuek M, Walsh ES, Leander KR, Howell BJ et al (2013) Endosomolytic bioreducible poly(amido amine disulfide) polymer conjugates for the in vivo systemic delivery of siRNA therapeutics. Bioconjug Chem 24:640–647
Saptarshi C, Ramakrishnan S (2011) Hyperbranched polyacetals with tunable degradation rates. Macromolecules 44:4658–4664
Chen H, Jia J, Duan X, Yang Z, Kong J (2015) Reduction-cleavable hyperbranched polymers with limited intramolecular cyclization via click chemistry. J Polym Sci PolChem 53:2374–2380
Acknowledgements
The financial support from National Natural Science Foundation of China (21374089/81400765) is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Duan, X., Wu, Y., Ma, M. et al. Amphiphilic polymer–drug conjugates based on acid-sensitive 100% hyperbranched polyacetals for cancer therapy. J Mater Sci 52, 9430–9440 (2017). https://doi.org/10.1007/s10853-017-1135-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1135-1