Abstract
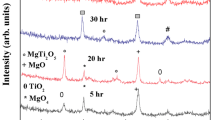
The reversible transition of wurtzite to rock salt phase under pressure is well reported in literature. The cubic phase is unstable under ambient conditions both in the bulk and in nanoparticles. This paper reports defect-induced stabilization of cubic ZnO phase in sub 20 nm ZnO particles and explores their optical properties. The size reduction was achieved by ball milling in a specially designed mill which allows a control of the milling temperature. The process of synthesis involved both variation of milling temperature (including low temperature ~150 K) and impact pressure. We show that these have profound influence in the introduction of defects and stabilization of the cubic phase. A molecular dynamics simulation is presented to explain the observed results. The measured optical properties have further supported the observations of defect-induced stabilization of cubic ZnO and reduction in particle size.






Similar content being viewed by others
References
Ozgur U, Alivov YI, Liu C, Teke A, Reshchikov MA, Dogan S, Avrutin V, Cho SJ, Morkoc H (2005) A comprehensive review of ZnO materials and devices. J Appl Phys 98:041301
Wang ZL (2004) Zinc oxide nanostructures: growth, properties and applications. J Phys 16:R829
Pearton SJ, Norton DP, Ip K, Heo YW, Steiner T (2005) Recent progress in processing and properties of ZnO. Prog Mater Sci 50:293–340
Decremps F, Datchi F, Saitta AM, Polian A, Pascarelli S, Dicicco A, Itie JP, Baudelet F (2003) Local structure of condensed zinc oxide. Phys Rev B 68:104101–1041010
Serrano J, Romero AH, Manjan FJ, Lauck R, Cardona M, Rubio A (2004) Pressure dependence of the lattice dynamics of ZnO: an ab initio approach. Phys Rev B 69:94306–94320
Gerward L, Olsen JS (1995) The high-pressure phase of zincite. J Synchrotron Radiat 2:233–235
Cai J, Chen N (2007) First-principles study of the wurtzite-to-rocksalt phase transition in zinc oxide. J Phys 19:266207
Solozhenko VL, Kurakevych OO, Sokolov PS, Baranov Andrey N (2011) Kinetics of the wurtzite-to-rock-salt phase transformation in ZnO at high pressure. J Phys Chem A 115:4354–4358
Wang S, Fan Z, Koster RS, Fang C, van Huis MA, Yalcin AO, Tichelaar FD, Zandbergen HW, Vlugt TJH (2014) New ab initio based pair potential for accurate simulation of phase transitions in ZnO. J Phys Chem C 118:11050–11061
Desgrenier S (1998) Structural and compressive parameters high-density phases of ZnO. Phys Rev B 58:14102–14115
Decremps F, Datchi F, Saitta AM, Polian A, Pascarelli S, Dicicco A, Itie IP, Baudelet F (2003) Local structure of condensed zinc oxide. Phys Rev B 68:104101
Baranov AN, Sokolov PS, Tafeenko VA, Lathe C, Zubavichus YV, Veligzhanin AA, Chukichev MV, Solozhenko VL (2013) Nanocrystallinity as a route to metastable phases: rock salt ZnO. Chem Mater 25:1775–1782
Gao Z, Gu Y, Zhang Y (2010) First-principles studies on the structural transition of ZnO nanowires at high pressure. J Nanomater 5:462032–462042
Kulkarni AJ, Zhou M, Sarasamak K, Limpijumnong S (2006) Novel phase transformation in ZnO nanowires under tensile loading. Phys Rev Lett 97:105502
Jiang Z, Olsen JS, Gerward L, Frost D, Rubie D, Peyronneau J (2000) Structural stability in nanocrystalline ZnO. Europhys Lett 50:48–52
Zhang L, Huang H (2007) Structural transformation of ZnO nanostructures. Appl Phys Lett 90:23115–23117
Dong Z, Zhuravlev KK, Morin SA, Li L, Jin S, Song Y (2012) Pressure-induced structural transformations of ZnO nanowires probed by X-ray diffraction. J Phys Chem C 116:2102–2107
Kotmool K, Bovornratanaraks T, Chakraborty S, Ahuja R (2015) The effect of morphology and confinement on the high-pressure phase transition in ZnO nanostructure. J Appl Phys 117:114309–114314
Wang L, Liu H, Qian J, Yang W, Zhao Y (2012) Structural stability and compressibility study for ZnO nanobelts under high pressure. J Phys Chem C 116:2074–2079
Tiwary CS, Kashyap S, Biswas K, Chattopadhyay K (2013) Synthesis of pure iron magnetic nanoparticles in large quantity. J Phys D 46:385001–385005
Verma A, Biswas K, Tiwary CS, Mondal AK, Chattopadhyay K (2011) Combined Cryo and room-temperature ball milling to produce ultrafine halide crystallites. Metall Mater Trans A 42:1127–1137
Tiwary CS, Verma A, Biswas K, Mondal AK, Chattopadhyay K (2011) Preparation of ultrafine CsCl crystallites by combined cryogenic and room temperature ball milling. Ceram Int 37:3677–3686
Tiwary CS, Saha S, Kumbhakar P, Chattopadhyay K (2014) Observation of combined effect of temperature and pressure on cubic to hexagonal phase transformation in ZnS at the nanoscale. Cryst Growth Design 14(9):4240–4246
Barai K, Tiwary CS, Chattopadhyay PP, Chattopadhyay K (2012) Synthesis of free standing nanocrystalline Cu by ball milling at cryogenic temperature. Mater Sci Eng A 558:52–58
Mohamed FA (2003) A dislocation model for the minimum grain size obtainable by milling. Acta Mater 51:4107–4119
Glushenkov AM, Zhang HZ, Chen Y (2008) Reactive ball milling to produce nanocrystalline ZnO. Mater Lett 62:4047–4049
Ao W, Li J, Yang H, Zhang X, Ma X (2006) Mechanochemical synthesis of zinc oxide nanocrystalline. Powder Technol 168:148–151
Tsuzuki T, Mccormick PG (2001) ZnO nanoparticles synthesised by mechanochemical processing. Scr Mater 44:1731–1734
Zhao DS, Zhao M, Jiang Q (2002) Size and temperature dependence of nanodiamond–nanographite transition related with surface stress. Diam Relat Mater 11:234–236
Bate CH, White WB, Roy R (1962) New high-pressure polymorph of zinc oxide. Science 137:993
Gilbert B, Huangs F, Lin Z, Goodell C, Zhang H, Banfield JF (2006) Surface chemistry controls crystallinity of ZnS nanoparticles. Nano Lett 6:605–610
Huang F, Banfield JF (2005) Size-dependent phase transformation kinetics in nanocrystalline ZnS. J Am Chem Soc 127:4523–4529
Li S, Lian JS, Jiang Q (2008) Modeling size and surface effects on ZnS phase selection. Chem Phys Lett 455:202–206
Liang T, Shan TR, Cheng YT, Devine BD, Noordhoek M, Li Y, Lu Z, Phillpot SR, Sinnott SB (2013) Classical atomistic simulations of surfaces and heterogeneous interfaces with the charge-optimized many body (COMB) potentials. Mater Sci Eng 74:255–279
Subramaniyan AK, Sun CT (2008) Continuum interpretation of virial stress in molecular simulations. Int J Solid Struct 45:4340–4346
Stuart SJ, Tutein AB, Harrison JA (2000) A reactive potential for hydrocarbons with intermolecular interactions. J Chem Phys 112:6472–6474
Rapaport DC (2004) The art of molecular dynamics, 2nd edn. Cambridge publishers, Cambridge, p 90
Chichvarina O, Herng TS, Phuah KC, Xiao W, Bao N, Feng YP, Ding J (2015) Stable zinc-blende ZnO thin films: formation and physical properties. J Mater Sci 50:28–33. doi:10.1007/s10853-014-8561-0
Jiang JZ (2004) Phase transformations in nanocrystals. J Mater Sci 39:5103–5110. doi:10.1023/B:JMSC.0000039191.87985.c1
Kakazey M, Vlasova M, Dominguez-Patino M, Leon I, Ristic M (2007) Reactionary processes during mechanical treatment of mixtures of ZnO and MnO2. I. Formation of defects and solid solution. J Mater Sci 42:7116–7122. doi:10.1007/s10853-007-1550-9
Chakraborty S, Tiwary CS, Kole AK, Kumbhakar P, Chattopadhyay K (2013) A simple method of synthesis and optical properties of Mn doped ZnO nanocups. Mater Lett 91:379–382
Kumbhakar P, Singh D, Tiwary CS, Mitra AK (2008) Chemical synthesis and visible photoluminescence emission from monodispersed ZnO nanoparticles. Chalcogenide Lett 5:387–394
Djurisic AB, Leung YH (2006) Optical properties of ZnO nanostructures. Small 2:944–961
Zeng H, Duan G, Li Y, Yang S, Xu X, Cai W (2010) Blue Luminescence of ZnO nanoparticles based on non-equilibrium processes: defect origins and emission controls. Adv Funct Mater 20:561–572
Fan XM, Lian JS, Zhao L, Liu YH (2005) Single violet luminescence emitted from ZnO films obtained by oxidation of Zn film on quartz glass. Appl Surf Sci 252:420–424
Zeng H, Yang S, Xu X, Cai W (2009) Dramatic excitation dependence of strong and stable blue luminescence of ZnO hollow nanoparticles. Appl Phys Lett 95:191904–191906
Xu PS, Sun YM, Shi CS, Xu FQ, Pan HB (2003) The electronic structure and spectral properties of ZnO and its defects. Nucl Instrum Methods Phys Res B 199:286–290
Acknowledgements
The authors would like to acknowledge the electron microscopy facilities available at the Advanced Facility for Microscopy and Microanalysis, Indian Institute of Science (IISc), Bangalore, India. The authors are grateful to UGC-NRCM, IISc, Bangalore for the partial financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tiwary, C.S., Vishnu, D., Kole, A.K. et al. Stabilization of the high-temperature and high-pressure cubic phase of ZnO by temperature-controlled milling. J Mater Sci 51, 126–137 (2016). https://doi.org/10.1007/s10853-015-9394-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9394-1