Abstract
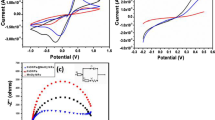
Laminar nanocomposite of α-ZrP/MnTMPyP, [5, 10, 15, 20-tetrakis (N-methylpyridinium-4-yl) porphyrinato manganese (III)], was obtained through the self-assembly of α-ZrP nanosheets and manganese porphyrin molecules, namely the exfoliation/restacking route. The final products were characterized by several analytic techniques such as XRD, IR, UV–Vis, and SEM. Meanwhile, the surface charge change of layered zirconium phosphate during the restacking process was monitored by a Zetasizer Nano instrument. The zeta potential value of α-ZrP colloidal dispersion is −40.1 mV, indicating that the colloidal dispersion was stable and well dispersed. The cyclic voltammetry measurements of α-ZrP/MnTMPyP film-modified glass carbon electrode displayed a pair of well-defined oxidation/reduction peaks with redox potentials at −0.256 and −0.197 V with an increase in the peak current compared to MnTMPyP aqueous solution. Furthermore, α-ZrP/MnTMPyP hybrid thin film exhibited excellent electrocatalytic activities toward oxidation of nitrite. The oxidation peak current increased linearly with the square root of scan rate, suggesting that the electrocatalytic process was controlled by nitrite diffusion. Finally, a detection limit of 5.3 × 10−5 M was estimated at a signal-to-noise ratio of 3.0 with a concentration range of 1.5 × 10−4 to 4.76 × 10−3 M.












Similar content being viewed by others
References
Tong Z et al (2006) Photoresponsive multilayer spiral nanotubes: intercalation of polyfluorinated cationic azobenzene surfactant into potassium niobate. J Am Chem Soc 128:684–685
Hosogi Y, Kato H, Kudo A (2008) Photocatalytic activities of layered titanates and niobates ion-exchanged with Sn2+ under visible light irradiation. J Phys Chem C 112:17678–17682
Zhang X et al (2010) Intercalation of methylene blue into layered potassium titanoniobate KTiNbO5: characterization and electrochemical investigation. J Mater Sci 45:1604–1609. doi:10.1007/s10853-009-4134-z
Han J et al (2011) Layer-by-layer assembly of layered double hydroxide/cobalt phthalocyanine ultrathin film and its application for sensors. J Mater Chem 21:2126–2130
Ma J et al (2014) Facile assembly for fast construction of intercalation hybrids of layered double hydroxides with anionic metalloporphyrin. Dalton Trans 43:9909–9915
Clearfield A, Stynes JA (1964) The preparation of crystalline zirconium phosphate and some observations on its ion exchange behaviour. J Inorg Nucl Chem 26:117–129
Clearfield A (1984) Inorganic ion exchangers with layered structures. Ann Rev Mater Sci 14:205–229
Alberti G et al (1996) Layered and pillared metal (IV) phosphates and phosphonates. Adv Mater 8:291–303
Sun L et al (2005) Effect of crystallinity on the intercalation of monoamine in α-zirconium phosphate layer structure. Chem Mater 17:5606–5609
Xiao H et al (2015) Amine-intercalated α-zirconium phosphates as lubricant additives. Appl Surf Sci 329:384–389
Wang H et al (2005) Study on the intercalation and interlayer state of porphyrins into α-zirconium phosphate. J Incl Phenom Macro 52:247–252
Dilgin Y et al (2005) Photoelectrochemical investigation of methylene blue immobilised on zirconium phosphate modified carbon paste electrode in flow injection system. Anal Chim Acta 542:162–168
Liu Y et al (2008) Direct electron transfer of hemoglobin in layered α-zirconium phosphate with a high thermal stability. Anal Biochem 375:27–34
Kumar CV, Chaudhari A (2002) High temperature peroxidase activities of HRP and hemoglobin in the galleries of layered Zr (IV) phosphate. Chem Commun 20:2382–2383
Bhambhani A, Kumar CV (2006) Tuning the properties of Hb intercalated in the galleries of α-ZrP with ionic strength: improved structure retention and enhanced activity. Chem Mater 18:740–747
Díaz A et al (2010) Nanoencapsulation of insulin into zirconium phosphate for oral delivery applications. Biomacromolecules 11:2465–2470
Mosby BM et al (2013) Surface functionalization of zirconium phosphate nanoplatelets for the design of polymer fillers. ACS Appl Mater Interfaces 6:585–592
Mosby BM et al (2014) Designable architectures on nanoparticle surfaces: zirconium phosphate nanoplatelets as a platform for tetravalent metal and phosphonic acid assemblies. Langmuir 30:2513–2521
Troup JM, Clearfield A (1977) Mechanism of ion exchange in zirconium phosphates. 20. Refinement of the crystal structure of alpha-zirconium phosphate. Inorg Chem 16:3311–3314
Dias PM, De Faria DLA, Constantino VRL (2000) Spectroscopic studies on the interaction of tetramethylpyridylporphyrins and cationic clays. J Incl Phenom Macro 38:251–266
Bizeto MA, De Faria DLA, Constantino VRL (2002) Porphyrin intercalation into a layered niobate derived from K4Nb6O17. J Mater Sci 37:265–270. doi:10.1023/A:1013687825874
Tong Z, Shichi T, Takagi K (2002) Visible-light induced charge-separation between consecutively cast porphyrin and methyl viologen multilayered titanoniobate hybrid films. J Phys Chem B 106:13306–13310
Kameyama H et al (2006) Oxidation of cyclohexene with molecular oxygen catalyzed by cobalt porphyrin complexes immobilized on montmorillonite. J Mol Catal A-chem 258:172–177
Kaschak DM et al (1999) Photoinduced energy and electron transfer reactions in lamellar polyanion/polycation thin films: toward an inorganic “leaf”. J Am Chem Soc 121:3435–3445
Zhang X et al (2009) Preparation and electrochemical behavior of methylene blue intercalated into layered niobate K4Nb6O17. J Mater Sci 44:3020–3025. doi:10.1007/s10853-009-3398-7
Shao F et al (2013) Synthesis and electrochemical properties study of novel intercalation compound of KCa2Nb3O10 with cationic methylene blue. Micro Nano Lett 8:788–791
Guo X et al (2005) Synthesis of a novel super-microporous layered material and its catalytic application in the vapor-phase Beckmann rearrangement of cyclohexanone oxime. Micropor Mesopor Mat 80:269–274
Coleman JN et al (2011) Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 331:568–571
Liu Z et al (2006) Synthesis, anion exchange, and delamination of Co-Al layered double hydroxide: assembly of the exfoliated nanosheet/polyanion composite films and magneto-optical studies. J Am Chem Soc 128:4872–4880
Liu Z et al (2007) General synthesis and delamination of highly crystalline transition-metal-bearing layered double hydroxides. Langmuir 23:861–867
Wang Y et al (2014) Coassembly of exfoliated Ni–In LDHs nanosheets with DNA and infrared emissivity study. J Mater Sci 49:6944–6951. doi:10.1007/s10853-014-8399-5
Yu J et al (2015) One-step direct synthesis of layered double hydroxide single-layer nanosheets. Nanoscale 7:9448–9451
Oshima T et al (2015) Intercalation of highly dispersed metal nanoclusters into a layered metal oxide for photocatalytic overall water splitting. Angew Chem Int Edit 54:2698–2702
Zhai Z et al (2012) Novel mesoporous NiO/HTiNbO5 nanohybrids with high visible-light photocatalytic activity and good biocompatibility. Nanoscale 4:547–556
Zhang L et al (2013) S-doped HTiNbO5 nanosheets: a novel efficient visible-light photocatalyst. Chin J Catal 34:2089–2097
Takei T et al (2006) Anodic electrodeposition of highly oriented zirconium phosphate and polyaniline-intercalated zirconium phosphate films. J Am Chem Soc 128:16634–16640
Kaschak DM et al (1998) Chemistry on the edge: a microscopic analysis of the intercalation, exfoliation, edge functionalization, and monolayer surface tiling reactions of α-zirconium phosphate. J Am Chem Soc 120:10887–10894
Kim HN et al (1997) Characterization of zirconium phosphate/polycation thin films grown by sequential adsorption reactions. Chem Mater 9:1414–1421
Sun L et al (2007) Preparation of exfoliated epoxy/α-zirconium phosphate nanocomposites containing high aspect ratio nanoplatelets. Chem Mater 19:1749–1754
Zhang X et al (2014) A manganese porphyrin intercalated lanthanum niobic acid nanocomposite utilized for electrocatalytic oxidation of nitrite. ECS Electrochem Lett 3:H17–H19
Alberti G, Torracca E (1968) Crystalline insoluble salts of polybasic metals-II. Synthesis of crystalline zirconium or titanium phosphate by direct precipitation. J Inorg Nucl Chem 30:317–318
Ma J et al (2015) Sandwich-structured composite from the direct coassembly of layered titanate nanosheets and Mn porphyrin and its electrocatalytic performance for nitrite oxidation. Mater Lett 150:122–125
Park IY, Kuroda K, Kato C (1989) Preparation of a layered double hydroxide-porphyrin intercalation compound. Chem Lett 11:2057–2058
Barloy L et al (1992) Manganese porphyrins adsorbed or intercalated in different mineral matrices: preparation and compared properties as catalysts for alkene and alkane oxidation. Mater Sci Forum 91:838
Halma M et al (2009) Immobilization of anionic iron (III) porphyrins into ordered macroporous layered double hydroxides and investigation of catalytic activity in oxidation reactions. J Mol Catal A 310:42–50
Halma M et al (2008) Synthesis, characterization, and catalytic activity of anionic iron (III) porphyrins intercalated into layered double hydroxides. J Catal 257:233–243
Zhang X et al (2013) Electrochemical investigation of a novel metalloporphyrin intercalated layered niobate modified electrode and its electrocatalysis on ascorbic acid. J Solid State Electr 17:3177–3184
Armijo F et al (2007) Electrocatalytic oxidation of nitrite to nitrate mediated by Fe(III) poly-3-aminophenyl porphyrin grown on five different electrode surface. J Mol Catal A 268:148–154
Zuo G et al (2007) Study of orientation mode of cobalt-porphyrin on the surface of gold electrode by electrocatalytic dioxygen reduction. J Mol Catal A 269:46–52
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant Nos. 21401062, 21201070), Natural Science Fund of Jiangsu Province (BK20140447, BK20141247, SBK201220654), and University Science Research Project of Jiangsu Province (13KJB430005, 12KJD150001).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pan, B., Ma, J., Zhang, X. et al. A laminar nanocomposite constructed by self-assembly of exfoliated α-ZrP nanosheets and manganese porphyrin for use in the electrocatalytic oxidation of nitrite. J Mater Sci 50, 6469–6476 (2015). https://doi.org/10.1007/s10853-015-9205-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9205-8