Abstract
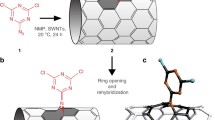
New hybrid halloysite nanotubes (HNTs) were obtained by grafting conjugated polyfluorenes (PFs) onto the surface via “click” chemistry. The hybrid material can be dispersed in organic solvent such as dichloromethane, and exhibits intense blue emission peaked at 448 nm. The absorption and emission spectra of the HNTs show distinct bathochromic shifts compared to PFs, which can be attributed to the extended π-conjugation as a result of the cycloaddition of azide and alkyne groups. The changes of the spectra also show that the PFs were grafted onto the HNTs through chemical bonds. Thermal gravimetric analyzer data show one more significant result, the grafting degree of the HNTs is 150 %, which means that a high mass ratio of PF polymers was grafted onto the HNTs. Furthermore, characterizations by infrared, nuclear magnetic resonance, and X-ray photoelectron spectroscopy also support the conclusion. The combination of HNTs with conjugated polymers will lead to a new generation of donor–acceptor nanohybrid materials which are promising in application in photoelectric fields.









Similar content being viewed by others
References
Dasgupta D, Shishmanova IK, Ruiz-Carretero A et al (2013) Patterned silver nanoparticles embedded in a nanoporous smectic liquid crystalline polymer network. J Am Chem Soc 135:10922–10925
Opanasenko M, Parker WON, Shamzhy M et al (2014) Hierarchical hybrid organic-inorganic materials with tunable textural properties obtained using zeolitic-layered precursor. J Am Chem Soc 136:2511–2519
Xia X, Chao D, Qi X et al (2013) Controllable growth of conducting polymers shell for constructing high-quality organic/inorganic core/shell nanostructures and their optical-electrochemical properties. Nano Lett 13:4562–4568
Kuila T, Bose S, Mishra AK, Khanra P, Kim NH, Lee JH (2012) Chemical functionalization of graphene and its applications. Prog Mater Sci 57:1061–1105
Lvov YM, Shchukin DG, Mohwald H, Price RR (2008) Halloysite clay nanotubes for controlled release of protective agents. ACS Nano 2:814–820
Abdullayev E, Sakakibara K, Okamoto K, Wei W, Ariga K, Lvov Y (2011) Natural tubule clay template synthesis of silver nanorods for antibacterial composite coating. ACS Appl Mater Inter 3:4040–4046
Fu XB, Ding Z, Zhang X et al (2014) Preparation of halloysite nanotube-supported gold nanocomposite for solvent-free oxidation of benzyl alcohol. Nanoscale Res Lett 9:282–285
Li C, Li X, Duan X, Li G, Wang J (2014) Halloysite nanotube supported Ag nanoparticles heteroarchitectures as catalysts for polymerization of alkylsilanes to superhydrophobic silanol/siloxane composite microspheres. J Colloid Interface Sci 436:70–76
Abdullayev E, Price R, Shchukin D, Lvov Y (2009) Halloysite tubes as nanocontainers for anticorrosion coating with benzotriazole. ACS Appl Mater Interfaces 1:1437–1443
Lu F, Gu L, Meziani MJ et al (2009) Advances in bioapplications of carbon nanotubes. Adv Mater 21:139–152
Popat KC, Eltgroth M, LaTempa TJ, Grimes CA, Desai TA (2007) Decreased Staphylococcus epidermis adhesion and increased osteoblast functionality on antibiotic-loaded titania nanotubes. Biomaterials 28:4880–4888
Lun H, Ouyang J, Yang H (2014) Natural halloysite nanotubes modified as an aspirin carrier. RSC Adv 4:44197–44202
Lee Y, Jung G-E, Cho SJ, Geckeler KE, Fuchs H (2013) Cellular interactions of doxorubicin-loaded DNA-modified halloysite nanotubes. Nanoscale 5:8577–8585
Pan J, Yao H, Xu L et al (2011) Selective recognition of 2,4,6-trichlorophenol by molecularly imprinted polymers based on magnetic halloysite nanotubes composites. J Phy Chem C 115:5440–5449
Kim SW, Kim T, Kim YS et al (2012) Surface modifications for the effective dispersion of carbon nanotubes in solvents and polymers. Carbon 50:3–33
Liu M, Jia Z, Jia D, Zhou C (2014) Recent advance in research on halloysite nanotubes-polymer nanocomposite. Prog Polym Sci 39:1498–1525
Li Z, Kulkarni SA, Boix PP et al (2014) Laminated carbon nanotube networks for metal electrode-free efficient perovskite solar cells. ACS Nano 8:6797–6804
Schnorr JM, Swager TM (2011) Emerging applications of carbon nanotubes. Chem Mater 23:646–657
Eder D (2010) Carbon nanotube-inorganic hybrids. Chem Rev 110:1348–1385
Karousis N, Tagmatarchis N, Tasis D (2010) Current progress on the chemical modification of carbon nanotubes. Chem Rev 110:5366–5397
Sahoo NG, Rana S, Cho JW, Li L, Chan SH (2010) Polymer nanocomposites based on functionalized carbon nanotubes. Prog Polym Sci 35:837–867
Spitalsky Z, Tasis D, Papagelis K, Galiotis C (2010) Carbon nanotube-polymer composites: chemistry, processing, mechanical and electrical properties. Prog Polym Sci 35:357–401
Du M, Guo B, Jia D (2010) Newly emerging applications of halloysite nanotubes: a review. Polym Int 59:574–582
Rawtani D, Agrawal YK (2012) Multifarious application of holloysite nanotubes: a review. Rev Adv Mater Sci 30:282–295
Yang S, Liu Z, Jiao Y, Liu Y, Ji C, Zhang Y (2014) New insight into PEO modified inner surface of HNTs and its nano-confinement within nanotube. J Mater Sci 49:4270–4278. doi:10.1007/s10853-014-8122-6
Alhuthali A, Low IM (2013) Water absorption, mechanical, and thermal properties of halloysite nanotube reinforced vinyl-ester nanocomposites. J Mater Sci 48:4260–4273. doi:10.1007/s10853-013-7240-x
Yah WO, Xu H, Soejima H, Ma W, Lvov Y, Takahara A (2012) Biomimetic dopamine derivative for selective polymer modification of halloysite nanotube lumen. J Am Chem Soc 134:12134–12137
Yuan P, Southon PD, Liu Z et al (2008) Functionalization of halloysite clay nanotubes by grafting with gamma-aminopropyltriethoxysilane. J Phy Chem C 112:15742–15751
Cai N, Dai Q, Wang Z, Luo X, Xue Y, Yu F (2015) Toughening of electrospun poly(l-lactic acid) nanofiber scaffolds with unidirectionally aligned halloysite nanotubes. J Mater Sci 50:1435–1445. doi:10.1007/s10853-014-8703-4
Cavallaro G, Lazzara G, Milioto S (2011) Dispersions of nanoclays of different shapes into aqueous and solid biopolymeric matrices. Extended physicochemical study. Langmuir 27:1158–1167
Cavallaro G, Lazzara G, Milioto S (2012) Exploiting the colloidal stability and solubilization ability of clay nanotubes/ionic surfactant hybrid nanomaterials. J Phys Chem C 116:21932–21938
Massaro M, Riela S, Lo Meo P et al (2014) Functionalized halloysite multivalent glycocluster as a new drug delivery system. J Mater Chem B 2:7732–7738
Shchukin DG, Sukhorukov GB, Price RR, Lvov YM (2005) Halloysite nanotubes as biomimetic nanoreactors. Small 1:510–513
Lvov Y, Abdullayev E (2013) Functional polymer–clay nanotube composites with sustained release of chemical agents. Prog Polym Sci 38:1690–1719
Yah WO, Takahara A, Lvov YM (2012) Selective modification of halloysite lumen with octadecylphosphonic acid: new inorganic tubular micelle. J Am Chem Soc 134:1853–1859
Riela S, Massaro M, Colletti CG et al (2014) Development and characterization of co-loaded curcumin/triazole-halloysite systems and evaluation of their potential anticancer activity. Int J Pharm 475:613–623
Yiu HHP, Wright PA (2005) Enzymes supported on ordered mesoporous solids: a special case of an inorganic-organic hybrid. J Mater Chem 15:3690–3700
Bruce IJ, Sen T (2005) Surface modification of magnetic nanoparticles with alkoxysilanes and their application in magnetic bioseparations. Langmuir 21:7029–7035
Beaupre S, Boudreault P-LT, Leclerc M (2010) Solar-energy production and energy-efficient lighting: photovoltaic devices and white-light-emitting diodes using poly(2,7-fluorene), poly(2,7-carbazole), and poly(2,7-dibenzosilole) derivatives. Adv Mater 22:E6–E27
Abbel R, Schenning APHJ, Meijer EW (2009) Fluorene-based materials and their supramolecular properties. J Polym Sci A 47:4215–4233
Tolman CA, Seidel WC, Gerlach DH (1972) Triarylphosphine and ethylene complexes of zerovalent nickel, palladium, and platinum. J Am Chem Soc 94:2669–2676
Luo Z, Song H, Feng X et al (2013) Liquid crystalline phase behavior and sol-gel transition in aqueous halloysite nanotube dispersions. Langmuir 29:12358–12366
Wan C, Li M, Bai X, Zhang Y (2009) Synthesis and characterization of photoluminescent Eu(III) coordination halloysite nanotube-based nanohybrids. J Phys Chem C 113:16238–16246
Denneval C, Moldovan O, Baudequin C et al (2013) Synthesis and photophysical properties of push-pull structures incorporating diazines as attracting part with a fluorene core. Eur J Org Chem 2013:5591–5602
Sandee AJ, Williams CK, Evans NR et al (2004) Solution-processible conjugated electrophosphorescent polymers. J Am Chem Soc 126:7041–7048
Houga C, Le Meins JF, Borsali R, Taton D, Gnanou Y (2007) Synthesis of ATRP-induced dextran-b-polystyrene diblock copolymers and preliminary investigation of their self-assembly in water. Chem Commun 29:3063–3065
Mulvihill MJ, Rupert BL, He R, Hochbaum A, Arnold J, Yang P (2005) Synthesis of bifunctional polymer nanotubes from silicon nanowire templates via atom transfer radical polymerization. J Am Chem Soc 127:16040–16041
Dax D, Xu C, Långvik O, Hemming J, Backman P, Willför S (2013) Synthesis of SET–LRP-induced galactoglucomannan-diblock copolymers. J Polym Sci A 51:5100–5110
Binder WH, Sachsenhofer R (2007) ‘Click’ chemistry in polymer and materials science. Macromol Rapid Commun 28:15–54
Moses JE, Moorhouse AD (2007) The growing applications of click chemistry. Chem Soc Rev 36:1249–1262
Acknowledgements
This work was supported by the National Science Foundation of China (Grant Nos. 201274037, 21304029 and 21474026), the Specialized Research Fund for the Doctoral Program of Higher Education (Grant no. 20121301120004), the Natural Science Foundation of Hebei Province (Grant No. B2013201117), the Project funded by China Postdoctoral Science Foundation (Grant No. 2014M551040), and the Foundation of Hebei Educational Committee (YQ2014025).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
10853_2015_8993_MOESM1_ESM.docx
Fig.S1-S7 show GPC elution curves of P2, 1H solid-state NMR spectra, and SEM micrograph of HNTs-P2. Supplementary material 1 (DOCX 14256 kb)
Rights and permissions
About this article
Cite this article
Zhang, H., Zhu, X., Wu, Y. et al. High-efficiency grafting of halloysite nanotubes by using π-conjugated polyfluorenes via “click” chemistry. J Mater Sci 50, 4387–4395 (2015). https://doi.org/10.1007/s10853-015-8993-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-8993-1