Abstract
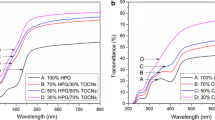
Nanofibrillated cellulose (NFC)-Norway spruce O-acetyl-galactoglucomannan (GGM) composite films were coated either with a novel succinic ester of GGM or with native GGM. NFC films were made for reference. The succinic ester of GGM was synthesised at low (GGM-Su1) and high (GGM-Su2) degree of substitution to obtain different level of water repellence. GGM and its succinic esters had good affinity with NFC substrate. This made it possible to implement the barrier functionality on the NFC network with the adequate mechanical properties. The coatings further enhanced the already excellent oxygen permeability properties, achieving 0.1 [(cm3 µm)(m2 kPa d)] as the lowest value with the NFC-GGM film double-coated with GGM-Su2. The films demonstrated pronounced stiffness by adding GGM to the NFC, as well as coating of GGM-Su2 on the NFC-GGM films at 0–90 % relative humidity. The films turned out to be impenetrable with grease even at high temperatures. NFC-GGM film with GGM-Su2 coating exhibited hydrophobic characteristics according to the water contact angle measurements. It was shown that adding 5.5 wt% of GGM to a NFC film and further 5.4 wt% of coating of GGM-Su or GGM on the film may highly enhance the feasibility of the biocomposites to be used for food packaging to replace typical oil-based non-biodegradable plastics currently used.
Graphical abstract








Similar content being viewed by others
References
Jayasiri HB, Purushothaman CS, Vennila A (2013) Quantitative analysis of plastic debris on recreational beaches in Mumbai, India. Mar Pollut Bull 77:107–112. doi:10.1016/j.marpolbul.2013.10.024
Thamae T, Bailie C (2008) Natural fibre composites, turning waste into useful materials. VDM Verlag Dr Muller Aktiengesellschaft & Co. Kg, pp 1–7
Willför S, Sjöholm R, Laine C et al (2003) Characterisation of water-soluble galactoglucomannans from Norway spruce wood and thermomechanical pulp. Carbohydr Polym 52:175–187
Song T, Pranovich A, Sumerskiy I, Holmbom B (2008) Extraction of galactoglucomannan from spruce wood with pressurised hot water. Holzforschung 62:659–666. doi:10.1515/HF.2008.131
Al Manasrah M, Kallioinen M, Ilvesniemi H, Maenttaeri M (2012) Recovery of galactoglucomannan from wood hydrolysate using regenerated cellulose ultrafiltration membranes. Bioresour Technol 114:375–381. doi:10.1016/j.biortech.2012.02.014
Rissanen JV, Grenman H, Xu C et al (2014) Obtaining spruce hemicelluloses of desired molar mass by using pressurized hot water extraction. ChemSusChem 7:2947–2953. doi:10.1002/cssc.201402282
Krogell J, Eranen K, Granholm K et al (2014) High-temperature pH measuring during hot-water extraction of hemicelluloses from wood. Ind Crop Prod 61:9–15. doi:10.1016/j.indcrop.2014.06.046
Kisonen V, Eklund P, Auer M et al (2012) Hydrophobication and characterisation of O-acetyl-galactoglucomannan for papermaking and barrier applications. Carbohydr Res 352:151–158. doi:10.1016/j.carres.2012.01.005
Mikkonen KS, Schmidt J, Vesterinen A-H, Tenkanen M (2013) Crosslinking with ammonium zirconium carbonate improves the formation and properties of spruce galactoglucomannan films. J Mater Sci 48:4205–4213. doi:10.1007/s10853-013-7233-9
Oinonen P, Areskogh D, Henriksson G (2013) Enzyme catalyzed cross-linking of spruce galactoglucomannan improves its applicability in barrier films. Carbohydr Polym 95:690–696. doi:10.1016/j.carbpol.2013.03.016
Kisonen V, Xu C, Eklund P et al (2014) Cationised O-acetyl galactoglucomannans: synthesis and characterisation. Carbohydr Polym 99:755–764. doi:10.1016/j.carbpol.2013.09.009
Lozhechnikova A, Dax D, Vartiainen J et al (2014) Modification of nanofibrillated cellulose using amphiphilic block-structured galactoglucomannans. Carbohydr Polym 110:163–172. doi:10.1016/j.carbpol.2014.03.087
Dax D, Eklund P, Hemming J et al (2013) Amphiphilic spruce galactoglucomannan derivatives based on naturally-occurring fatty acids. BioResources 8:3771–3790. doi:10.15376/biores.8.3.3771-3790
Mikkonen KS, Stevanic JS, Joly C et al (2011) Composite films from spruce galactoglucomannans with microfibrillated spruce wood cellulose. Cellulose 18:713–726. doi:10.1007/s10570-011-9524-0
Trovatti E, Fernandes SCM, Rubatat L et al (2012) Pullulan–nanofibrillated cellulose composite films with improved thermal and mechanical properties. Compos Sci Technol 72:1556–1561. doi:10.1016/j.compscitech.2012.06.003
Syverud K, Stenius P (2009) Strength and barrier properties of MFC films. Cellulose 16:75–85. doi:10.1007/s10570-008-9244-2
Besbes I, Vilar MR, Boufi S (2011) Nanofibrillated cellulose from alfa, eucalyptus and pine fibres: preparation, characteristics and reinforcing potential. Carbohydr Polym 86:1198–1206. doi:10.1016/j.carbpol.2011.06.015
Okuba K, Fujii T, Yamashita N (2005) Improvement of interfacial adhesion in bamboo polymer composite enhanced with micro-fibrillated cellulose. JSME Int J Ser Solid Mech Mater Eng 48:199–204
Zhang Z, Sèbe G, Rentsch D et al (2014) Ultralightweight and flexible silylated nanocellulose sponges for the selective removal of oil from water. Chem Mater 26:2659–2668. doi:10.1021/cm5004164
Xhanari K, Syverud K, Chinga-Carrasco G et al (2011) Structure of nanofibrillated cellulose layers at the o/w interface. J Colloid Interface Sci 356:58–62. doi:10.1016/j.jcis.2010.12.083
Vuoti S, Talja R, Johansson L-S et al (2013) Solvent impact on esterification and film formation ability of nanofibrillated cellulose. Cellulose 20:2359–2370. doi:10.1007/s10570-013-9983-6
Österberg M, Vartiainen J, Lucenius J et al (2013) A fast method to produce strong NFC films as a platform for barrier and functional materials. ACS Appl Mater Interfaces 5:4640–4647. doi:10.1021/am401046x
Zhou Q, Greffe L, Baumann MJ et al (2005) Use of xyloglucan as a molecular anchor for the elaboration of polymers from cellulose surfaces: a general route for the design of biocomposites. Macromolecules 38:3547–3549. doi:10.1021/ma047712k
Stevanic JS, Mikkonen KS, Xu C et al (2014) Wood cell wall mimicking for composite films of spruce nanofibrillated cellulose with spruce galactoglucomannan and arabinoglucuronoxylan. J Mater Sci 49:5043–5055. doi:10.1007/s10853-014-8210-7
Escalante A, Gonçalves A, Bodin A et al (2012) Flexible oxygen barrier films from spruce xylan. Carbohydr Polym 87:2381–2387. doi:10.1016/j.carbpol.2011.11.003
Isogai A (2013) Wood nanocelluloses: fundamentals and applications as new bio-based nanomaterials. J Wood Sci 59:449–459. doi:10.1007/s10086-013-1365-z
Henriksson M, Henriksson G, Berglund LA, Lindström T (2007) An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur Polym J 43:3434–3441. doi:10.1016/j.eurpolymj.2007.05.038
Henriksson M, Berglund LA, Isaksson P et al (2008) Cellulose nanopaper structures of high toughness. Biomacromolecules 9:1579–1585. doi:10.1021/bm800038n
Sehaqui H, Zhou Q, Ikkala O, Berglund LA (2011) Strong and tough cellulose nanopaper with high specific surface area and porosity. Biomacromolecules 12:3638–3644. doi:10.1021/bm2008907
T 454 om-94 Turperntine test for voids in glassine and greaseproof papers (2000)
Bollstrom R, Saarinen JJ, Raty J, Toivakka M (2012) Measuring solvent barrier properties of paper. Meas Sci Technol 23:015601. doi:10.1088/0957-0233/23/1/015601
Kisonen V, Xu C, Bollström R et al (2014) O-acetyl galactoglucomannan esters for barrier coatings. Cellulose 21:4497–4509. doi:10.1007/s10570-014-0428-7
Hannuksela T, Hervé du Penhoat C (2004) NMR structural determination of dissolved O-acetylated galactoglucomannan isolated from spruce thermomechanical pulp. Carbohydr Res 339:301–312. doi:10.1016/j.carres.2003.10.025
Ekholm FS, Ardá A, Eklund P et al (2012) Studies related to Norway spruce galactoglucomannans: chemical synthesis, conformation analysis, NMR spectroscopic characterization, and molecular recognition of model compounds. Chemistry 18:14392–14405. doi:10.1002/chem.201200510
Yuan Y, Lee TR (2013) Contact angle and wetting properties. In: Bracco G, Holst B (eds) Surface science techniques. Springer, Berlin, Heidelberg, pp 3–34
Spiridon I, Teacă C-A, Bodîrlău R, Bercea M (2013) Behavior of cellulose reinforced cross-linked starch composite films made with tartaric acid modified starch microparticles. J Polym Environ 21:431–440. doi:10.1007/s10924-012-0498-2
Kwak S-Y, Jung SG, Kim SH (2001) Structure-motion-performance relationship of flux-enhanced reverse osmosis (RO) membranes composed of aromatic polyamide thin films. Environ Sci Technol 35:4334–4340. doi:10.1021/es010630g
Hartman J, Albertsson A-C, Sjöberg J (2006) Surface- and bulk-modified galactoglucomannan hemicellulose films and film laminates for versatile oxygen barriers. Biomacromolecules 7:1983–1989. doi:10.1021/bm060129m
Kjellgren H, Gaellstedt M, Engstroem G, Jaernstroem L (2006) Barrier and surface properties of chitosan-coated greaseproof paper. Carbohydr Polym 65:453–460. doi:10.1016/j.carbpol.2006.02.005
Mikkonen KS, Heikkinen S, Soovre A et al (2009) Films from oat spelt arabinoxylan plasticized with glycerol and sorbitol. J Appl Polym Sci 114:457–466. doi:10.1002/app.30513
Kisonen V, Xu C, Böllstrom R et al (2014) O-acetyl galactoglucomannan esters for barrier coatings. Cellul Dordr Neth 21:4497–4509. doi:10.1007/s10570-014-0428-7
Aulin C, Gällstedt M, Lindström T (2010) Oxygen and oil barrier properties of microfibrillated cellulose films and coatings. Cellulose 17:559–574. doi:10.1007/s10570-009-9393-y
Aulin C, Karabulut E, Tran A et al (2013) Transparent nanocellulosic multilayer thin films on polylactic acid with tunable gas barrier properties. ACS Appl Mater Interfaces 5:7352–7359. doi:10.1021/am401700n
Hansen NML, Blomfeldt TOJ, Hedenqvist MS, Plackett DV (2012) Properties of plasticized composite films prepared from nanofibrillated cellulose and birch wood xylan. Cellul Dordr Neth 19:2015–2031. doi:10.1007/s10570-012-9764-7
Mikkonen KS, Tenkanen M (2012) Sustainable food-packaging materials based on future biorefinery products: xylans and mannans. Trends Food Sci Technol 28:90–102. doi:10.1016/j.tifs.2012.06.012
Van Tuil R, Fowler P, Lawther M, Weber CJ (2000) Properties of biobased packaging materials. In Biobased packaging materials for the food industry—Status and perspectives. KVL, Frederiksberg, pp 8–33
Eronen P, Österberg M, Heikkinen S et al (2011) Interactions of structurally different hemicelluloses with nanofibrillar cellulose. Carbohydr Polym 86:1281–1290. doi:10.1016/j.carbpol.2011.06.031
Hansen NML, Plackett D (2008) Sustainable films and coatings from hemicelluloses: a review. Biomacromolecules 9:1493–1505. doi:10.1021/bm800053z
Acknowledgements
This work was carried out in framework of the Future Biorefinery Project by the Finnish Funding Agency for Technology and Innovation and Fibic Ltd. This work was part of the activities of the Åbo Akademi Process Chemistry Centre and Bioregs graduate school. This work made use of Aalto University Bioeconomy Facilities. We thank the staff of Metla in Vantaa and Lappeenranta University of Technology for providing the filtrated GGM, Hanna Lindqvist of our laboratory and Maristiina Nurmi of the Laboratory of Paper Coating and Converting for the practical help. The consultation on the concept by Lars Berglund of KTH, is highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kisonen, V., Prakobna, K., Xu, C. et al. Composite films of nanofibrillated cellulose and O-acetyl galactoglucomannan (GGM) coated with succinic esters of GGM showing potential as barrier material in food packaging. J Mater Sci 50, 3189–3199 (2015). https://doi.org/10.1007/s10853-015-8882-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-8882-7