Abstract
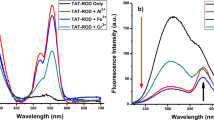
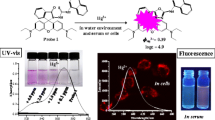
A variety of chemosensors have been reported for detection of metal ions. However, the metal ions could not be separated and removed at the same time for the goal of water purification. This paper presents to detect and remove metal ions from aqueous solution simultaneously by a fluorescence chemosensor and functional magnetic nanoparticles. A novel probe adamantyl (AD)–maleic anhydride (MAH)–rhodamine B (RhB) was designed and synthesized from RhB, ethylene diamine, MAH, and AD. AD–MAH–RhB showed high selectivity and sensitivity to metal ions in aqueous solution. The sensing mechanism was explored by FTIR and mass spectra. The results suggested that AD–MAH–RhB could conjugate with metal ions and form the binding complexes with various stoichiometries of probe and metal ions. Moreover, β-cyclodextrin-modified magnetic nanoparticles (CD-MNP) were fabricated and used as host materials to form inclusion complex of CD-MNP and AD–MAH–RhB-metal ions. Then, the metal ions could be removed by an outer magnet, which were confirmed by fluorescent spectrum. The probe and CD-MNP had the great potential application for sewage treatment.










Similar content being viewed by others
References
Prodi L, Bargossi C, Montalti M, Zaccheroni N, Su N, Bradshaw JS, Izatt RM, Savage PB (2000) An effective fluorescent chemosensor for mercury ions. J Am Chem Soc 122:6769–6770
Kim HN, Ren WX, Kim JS, Yoon J (2012) Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem Soc Rev 41:3210–3244
Yean LP, Ahmad ZA (2012) Effect of low Fe3+ doping on characteristics, sonocatalytic activity and reusability of TiO2 nanotubes catalysts for removal of Rhodamine B from water. Hazardous Mater 235–236:326–335
Xu JH, Hou YM, Ma QJ, Wu XF, Wei XJ (2013) A highly selective fluorescent sensor for Fe3+ based on covalently immobilized derivative of naphthalimide. Spectrochim Acta A112:116–124
Ho JA, Chang HC, Su WT (2012) DOPA-mediated reduction allows the facile synthesis of fluorescent gold nanoclusters for use as sensing probes for ferric ions. Anal Chem 84:3246–3253
Li YN, Huang H, Li Y, Su XG (2013) Highly sensitive fluorescent sensor for mercury (II) ion based on layer-by-layer self-assembled films fabricated with water-soluble fluorescent conjugated polymer. Sensor Actuat B 188:772–777
Li JF, Wu YZ, Song FY, Wei G, Cheng YX, Zhu CJ (2012) A highly selective and sensitive polymer-based OFF–ON fluorescent sensor for Hg2+ detection incorporating salen and perylenyl moieties. J Mater Chem 22:478–482
Wan XJ, Yao S, Liu HY, Yao YW (2013) Selective fluorescence sensing of Hg2+ and Zn2+ ions through dual independent channels based on the site-specific functionalization of mesoporous silica nanoparticles. J Mater Chem A 1:10505–10512
Kyzas GZ, Travlou NA, Deliyanni EA (2014) The role of chitosan as nanofiller of graphite oxide for the removal of toxic mercury ions. Colloids Surf B 113:467–476
Zhang D, Zou RY, Wan M, Chai MM, Wang XB, Ye Y, Zhao YF (2013) Novel series colorimetric and Off–On fluorescent chemosensors for Fe3+ based on Rhodamine B derivative. J Fluoresc 23:13–19
Chai MM, Zhang D, Wang M, Hong HJ, Yong Y, Zhao YF (2012) Four rhodamine B-based fluorescent chemosensor for Fe3+ in aqueous solution. Sensor Actuat B 174:231–236
Zhang LB, Li T, Li BL, Li J, Wang EK (2010) Carbon nanotube–DNA hybrid fluorescent sensor for sensitive and selective detection of mercury(II) ion. Chem Commun 46:1476–1478
Kim I, Kim D, Sambasivan S, Ahn KH (2012) Synthesis of π-extended coumarins and evaluation of their precursors as reactive fluorescent probes for mercury ions. Asian J Org Chem 1:60–64
Mu HL, Gong R, Ma Q (2007) A novel colorimetric and fluorescent chemosensor: synthesis and selective detection for Cu2+ and Hg2+. Tetrahedron Lett 48:5525–5529
Jk Ni, Li QY, Li B, Zhang LM (2013) A novel fluorescent probe based on Rhodamine B derivative for highly selective and sensitive detection of mercury(II) ion in aqueous solution. Sensor Actuat B 186:278–285
Wu C, Bian QN, Zhang BG, Cai X, Zhang SD, Zheng H, Yang SY, Jiang YB (2012) Ring expansion of spiro-thiolactam in rhodamine scaffold: switching the recognition preference by adding one atom. Org Lett 4:4198–4201
Wang W, Zhang Y, Yang QB, Sun MD, Fei XL, Song Y, Zhang YM, Li YX (2013) Fluorescent and colorimetric magnetic microspheres as nanosensors for Hg2+ in aqueous solution prepared by a sol–gel grafting reaction and host–guest interaction. Nanoscale 5:4958–4965
Luo J, Jiang SS, Qin SH, Wu HQ, Wang Y, Jiang JQ, Liu XY (2011) Highly sensitive and selective turn-on fluorescent chemosensor for Hg2+ in pure water based on a rhodamine containing water-soluble copolymer. Sensor Actuat B 160:1191–1197
Yang H, Zhou ZG, Huang KW, Yu MX, Li FY, Yi T, Huang CH (2007) Multisignaling optical-electrochemical sensor for Hg2+ based on a rhodamine derivative with a ferrocene unit. Org Lett 9:4729–4732
Wu DY, Huang W, Lin ZH, Duan CY, He C, Wu S, Wang DH (2008) Highly sensitive multiresponsive chemosensor for selective detection of Hg2+ in natural water and different monitoring environments. Inorg Chem 47:7190–7201
Barman S, Sadhukhan M (2012) Facile bulk production of highly blue fluorescent graphitic carbon nitride quantum dots and their application as highly selective and sensitive sensors for the detection of mercuric and iodide ions in aqueous media. J Mater Chem 22:21832–21837
Abbaspour A, Noori A (2011) A cyclodextrin host-guest recognition approach to an electrochemical sensor for simultaneous quantification of serotonin and dopamine. Biosens Bioelectron 26:4674–4680
Badruddoza AZM, Rahman MT, Ghosh S, Hossain MZ, Shi JZ, Hidajat K, Uddin MS (2013) Beta-cyclodextrin conjugated magnetic, fluorescent silica core–shell nanoparticles for biomedical applications. Carbohyd Polym 95:449–457
Liu JC, Liu R, Jiang JQ, Liu XY (2013) Design and synthesis of water-soluble photosensitive cyclodextrin and its application in dispersing carbon nanotubes. J Appl Polym Sci 130:2588–2593
Xu MY, Wu SZ, Zeng F, Yu CM (2010) Cyclodextrin supramolecular complex as a water-soluble ratiometric sensor for ferric ion sensing. Langmuir 26:4529–4534
Wang YP, Ma N, Wang ZQ, Zhang X (2007) Photocontrolled reversible supramolecular assemblies of an azobenzene-containing surfactant with alpha-cyclodextrin. Angew Chem Int Ed 46:2823–2826
Xie J, Yin YX, Jin GL, He MX, Diao GW (2011) Theoretical study on the inclusion complex of β-cyclodextrin and nitrobenzene. Chinese J Struc Chem 30:706–716
Wu YP, Zuo F, Zheng ZH, Ding XB, Peng YX (2009) A novel approach to molecular recognition surface of magnetic nanoparticles based on host–guest effect. Nanoscale Res Lett 4:738–747
Ghosh S, Badruddoza AZM, Uddin MS, Hidajat K (2011) Adsorption of chiral aromatic amino acids onto carboxymethyl-β-cyclodextrin bonded Fe3O4/SiO2 core–shell nanoparticles. J Colloid Interface Sci 354:483–492
Huan XY, Wang DL, Dong RJ, Tu CL, Zhu BS, Yan DY, Zhu XY (2012) Supramolecular ABC miktoarm star terpolymer based on host–guest inclusion complexation. Macromolecules 45:5941–5947
Ma LJ, Li Y, Li L, Wu YQ, Buchet R, Ding YH (2009) Larification of the binding model of lead(II) with a highly sensitive and selective fluoroionophore sensor by spectroscopic and structural study. Spectrochim Acta A 72:306–311
Berezovskaya Y, Armstrong CT, Boyle AL, Porrini M, Woolfson DN, Barran PE (2011) Metal binding to a zinc-finger peptide: a comparison between solution and the gas phase. Chem Commun 47:412–414
Qin W, Zou B, Zhang Y, Yi XH, Pan ZH (2013) UV–VIS, fluorescence and mass spectrometry investigation on the transition metal ion chelation of two bioisosteres of resveratrol. Asian J Chem 25:2185–2188
Yang XF, Liu P, Wang LP, Zhao ML (2008) A chemosensing ensemble for the detection of cysteine based on the inner filter effect using a Rhodamine B spirolactam. J Fluoresc 18:453–459
Bhalla V, Tejpal R, Kumar M (2010) Rhodamine appended terphenyl: a reversible “off–on” fluorescent chemosensor for mercury ions. Sensor Actuat B 151:180–185
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 21004029), MOE & SAFEA for the 111 Project (B13025) and Jiangsu Key Laboratory of Advanced Functional Polymer Design and Application (Soochow University, KJS1141).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shi, D., Ni, M., Zeng, J. et al. Simultaneous detection and removal of metal ions based on a chemosensor composed of a rhodamine derivative and cyclodextrin-modified magnetic nanoparticles. J Mater Sci 50, 168–175 (2015). https://doi.org/10.1007/s10853-014-8576-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8576-6