Abstract
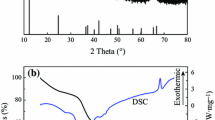
Manganese dioxide/multiwalled carbon nanotubes hybrid hollow microspheres were prepared via layer-by-layer assembly technique by alternately adsorbing aminated manganese dioxide (AMnO2) and carboxylated multiwalled carbon nanotubes (CMWCNTs) using polystyrene sulfonate microspheres as sacrificial templates. The structures and morphologies of the hybrid hollow microspheres were characterized by Fourier transform infrared spectroscopy, X-ray diffraction, transmission electron microscopy, and field emission scanning electron microscope. Capacitive properties of the prepared hybrid hollow microspheres were investigated by cyclic voltammetry, galvanostatic charge–discharge, and electrochemical impedance spectroscopy in a three-electrode experimental setup using 1.0 mol L−1 Na2SO4 solution as electrolyte. The result showed that the specific capacitance increased with the increase in the content of AMnO2 and CMWCNTs, which reached 169 F g−1 at a current density of 0.5 A g−1 when AMnO2 and CMWCNTs alternately adsorbed for ten times. And the capacitance retention was about 82 % after 800 times of cyclic voltammetry tests at a scan rate of 80 mV s−1.








Similar content being viewed by others
References
Simon P, Gogotsi Y (2008) Nat Mater 7:845
Yu GH, Xie X, Pan LJ, Bao ZN, Cui Y (2013) Nano Energy 2:213
Simon P, Gogotsi Y (2012) Acc Chem Res. doi:10.1021/ar200306b
Li Q, Liu JH, Zou JH, Chunder A, Chen YQ, Zhai L (2011) J Power Sources 196:565
Zhao X, Zhang LL, Murali S, Stoller MD, Zhang QH, Zhu YW, Ruoff RS (2012) ACS Nano 6:5404
Lei ZB, Shi FH, Lu L (2012) ACS Appl Mater Interfaces 4:1058
Fan ZJ, Xie MM, Jin X, Yan J, Wei T (2011) J Electroanal Chem 659:191
Sumboja A, Foo CY, Yan J, Yan CY, Gupta RK, Lee PS (2012) J Mater Chem 22:23921
Huo YQ, Zhang HY, Jiang JY, Yang Y (2012) J Mater Sci 47:7026. doi:10.1007/s10853-012-6654-1
Hou Y, Cheng YW, Hobson T, Liu J (2010) Nano Lett 10:2727
Guan C, Xia XH, Meng N, Zeng ZY, Cao XH, Soci C, Zhang H, Fan HJ (2012) Energy Environ Sci 5:9085
Zhao D, Guo XY, Gao Y, Gao F (2012) ACS Appl Mater Interfaces 4:5583
Mu B, Liu P, Wang AQ (2013) Electrochim Acta 88:177
Dubal DP, Holze P, Kulal PM (2013) J Mater Sci 48:714. doi:10.1007/s10853-012-6783-6
Lou XW, Archer LA, Yang ZC (2008) Adv Mater 20:3987
Lai XY, Halpert JE, Wang D (2012) Energy Environ Sci 5:5604
Xiang Y, Lu SF, Jiang SP (2012) Chem Soc Rev 41:7291
Lee JL, Ha JU, Choe SJ, Lee CS, Shim SE (2006) J Colloid Interface Sci 298:663
Ragupathy P, Park DH, Campet G, Vasan HN, Hwang SJ, Choy JH, Munichandraiah NM (2009) J Phys Chem C 113:6303
Yang SB, Feng XL, Ivanovici S, Müllen K (2010) Angew Chem Int Ed 49:8408
Teng F, Santhanagopalan S, Wang Y, Meng DD (2010) J Alloy Compd 499:259
Xia H, Wang Y, Lin JY, Lu L (2012) Nanoscale Res Lett 7:33
Zheng HJ, Wang JX, Jia Y, Ma CA (2012) J Power Sources 216:508
Li ZP, Wang JQ, Liu XH, Liu S, Ou JF, Yang SR (2011) J Mater Chem 21:3397
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, W., Mu, B. & Wang, A. Preparation of manganese dioxide/multiwalled carbon nanotubes hybrid hollow microspheres via layer-by-layer assembly for supercapacitor. J Mater Sci 48, 7581–7586 (2013). https://doi.org/10.1007/s10853-013-7574-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-013-7574-4