Abstract
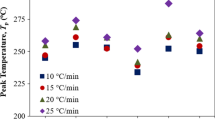
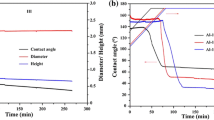
The wetting of (0001) α-alumina single crystals by Mg–Al alloys over a wide composition range at 1073 K was investigated using an improved sessile drop method in a flowing argon atmosphere. The initial contact angles are between 103° and 84°, almost linearly decreasing with increasing nominal Mg concentration, suggesting that the addition of Mg to Al improves the initial wettability. According to the evolution of contact angle and contact diameter, representative stages were identified to characterize the complex wetting behavior in the presence of evaporation. The wetting kinetics was dependent on the nominal Mg concentration in the alloy. Two patterns of “stick–slip” behavior were observed in the wetting process and interpreted by combining the effects of interfacial reaction and evaporation of magnesium. In addition, the dependence of the interfacial reaction on the Mg–Al alloy concentration was thermodynamically analyzed. The dominant reaction product at 1073 K should be MgO when x Mg > 9 mol%, while MgAl2O4 when x Mg < 9 mol%. However, because of the continuous consumption of Mg due to the evaporation and reaction, its concentration in the alloy progressively decreased with time. As a result, MgO formed usually earlier while MgAl2O4 later even for the alloys with higher than 9 mol% Mg.







Similar content being viewed by others
References
Delannay F, Froyen L, Deruyttere A (1987) J Mater Sci 22:1. doi:10.1007/BF01160545
Shen P, Zhang D, Lin QL, Shi LX, Jiang QC (2010) Mater Chem Phys 122:290
Fujii H, Izutani S, Matsumoto T, Kiguchi S, Nogi K (2006) Mater Sci Eng, A 417:99
Shi LX, Shen P, Zhang D, Jiang QC (2011) Mater Chem Phys 130:1125
Carnahan RD, Johnston TL, Li CH (1958) J Am Ceram Soc 41:343
Champion JA, Keene BJ, Sillwood JM (1969) J Mater Sci 4:39. doi:10.1007/BF00555046
John H, Hausner H (1986) J Mater Sci Lett 5:549
Ip SW, Kucharski M, Toguri JM (1993) J Mater Sci Lett 12:1699
Zhou XB, De Hosson JThM (1995) J Mater Sci 30:3571. doi:10.1007/BF00351867
Levi G, Kaplan WD (2002) Acta Mater 50:75
Saiz E, Tomsia AP, Cannon RM (1998) Acta Mater 46:2349
Saiz E, Tomsia AP, Suganuma K (2003) J Eur Ceram Soc 23:2787
Ksiazek M, Sobczak N, Mikulowski B, Radziwill W, Surowiak I (2002) Mater Sci Eng, A 324:162
Jonas TR, Cornie JA, Russell KC (1995) Metall Mater Trans A 26:1491
Aguilar-Santillan J (2010) Metall Mater Trans A 41:676
Laurent V, Chatain D, Chatillon C, Eustathopoulos N (1988) Acta Metall 36:1797
Shen P, Fujii H, Matsumoto T, Nogi K (2003) Acta Mater 51:4897
Brennan JJ, Pask JA (1968) J Am Ceram Soc 51:569
Weirauch DA Jr (1988) J Mater Res 3(4):729
Klinter AJ, Mendoza-Suarez G, Drew RAL (2008) Mater Sci Eng, A 495:147
Shen P, Fujii H, Matsumoto T, Nogi K (2004) J Am Ceram Soc 87:2151
Barin I (1995) Thermochemical data of pure substances, 3rd edn. Wiley-VCH Verlag GmbH, Weinheim
McLeod AD, Gabryel CM (1992) Metall Mater Trans A 23:1279
Massalski TB (1996) Binary alloys phase diagrams. ASM International, (CD–ROM)
Hultgren R, Desai PD, Hawkins DT, Gleiser M, Kelley KK (1973) Selected values of the thermodynamic properties of binary alloys. ASM, Metals Park, OH
Zhong WM, L’espérance G, Suétry M (1995) Metall Mater Trans A 26:2625
Hallestedt B, Liu ZK, Agren J (1990) Mater Sci Eng, A 129:135
Iida T, Guthrie RIL (1993) The physical properties of liquid metals. Clarendon Press, Oxford
Eustathopoulos N (1998) Acta Mater 46:2319
Zhang D (2012) Wetting of ceramics by molten Mg and effect of evaporation. PhD thesis, Jilin University, China (see the supplementary material)
Shen P, Fujii H, Matsumoto T, Nogi K (2004) Acta Mater 52:887
Mullins WW (1958) Acta Metall 6:414
Nowak N (2010) PhD thesis, Foundry Research Institute, Krakow, Poland
Lea C, Molinari C (1984) J Mater Sci 19:2336. doi:10.1007/BF01058110
Keene BJ (1993) Int Mater Rev 38:157
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 50871045), National Basic Research Program of China (973 program) (No. 2012CB619600), Key Project of Chinese Ministry of Education (No. 108043) and Graduate Innovation Fund of Jilin University (No. 20121086).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, L., Shen, P., Zhang, D. et al. Wetting of (0001) α-alumina single crystals by molten Mg–Al alloys in the presence of evaporation. J Mater Sci 47, 8372–8380 (2012). https://doi.org/10.1007/s10853-012-6784-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-012-6784-5