Abstract
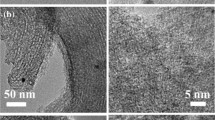
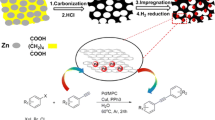
Heterogeneous palladium catalysts have been supported on the ordered mesoporous carbons (Pd/OMC) with bimodal pores which are prepared by the surfactant-templating approach. Characterization using XRD, TEM, XPS, H2 chemisorption, and N2 sorption techniques reveals that the Pd/OMC catalysts have the ordered 2-D hexagonal mesostructure (space group of p6mm), extremely high surface areas (~1800 m2/g), large pore volumes (~1.64 cm3/g), bimodal pores (6.3 nm of primary mesopores and 1.7 nm of secondary mesopores inside the pore walls), hydrophobic carbon surface, and small metal particles well-dispersed inside the secondary small mesopores. This catalyst exhibits a high yield of 43% for biphenyl from the Ullmann coupling reaction of chlorobenzene in water at 100 °C without assistance of any phase transfer catalyst and can be reused up to 10 times, providing potential opportunities for industrial applications such as coupling and hydrogenation reactions.








Similar content being viewed by others
References
Balachari D, Quinn L, O’Doherty GA (1999) Tetrahedron Lett 40:4769
Kohler K, Heidenreich RG, Krauter JGE, Pietsch M (2002) Chem Eur J 8:622
Yin LX, Liebscher J (2007) Chem Rev 107:133
Kyotani T (2000) Carbon 38:269
Lee J, Kim J, Hyeon T (2006) Adv Mater 18:2073
Yang H, Zhao DY (2005) J Mater Chem 15:1217
Tiemann M (2008) Chem Mater 20:961
Ryoo R, Joo SH, Kruk M, Jaroniec M (2001) Adv Mater 13:677
Wan Y, Yang HF, Zhao DY (2006) Acc Chem Res 39:423
Lu AH, Schuth F (2006) Adv Mater 18:1793
Liang CD, Hong KL, Guiochon GA, Mays JW, Dai S (2004) Angew Chem Int Ed 43:5785
Tanaka S, Nishiyama N, Egashira Y, Ueyama K (2005) Chem Commun 212:5
Meng Y, Gu D, Zhang FQ, Shi YF, Yang HF, Li Z, Yu CZ, Tu B, Zhao DY (2005) Angew Chem Int Ed 44:7053
Wan Y, Qian XF, Jia NQ, Wang ZY, Li HX, Zhao DY (2008) Chem Mater 20:1012
Wan Y, Shi YF, Zhao DY (2008) Chem Mater 20:932
Hassan J, Sevignon M, Gozzi C, Schulz E, Lemaire M (2002) Chem Rev 102:1359
Venkatraman S, Li CJ (1999) Org Lett 1:1133
Mukhopadhyay S, Rothenberg G, Gitis D, Sasson Y (2000) Org Lett 2:211
Polshettiwar V, Molnar A (2007) Tetrahedron 63:6949
Davies IW, Matty L, Hughes DL, Reider PJ (2001) J Am Chem Soc 123:10139
Li CJ (2005) Chem Rev 105:3095
Wu XF, Li XH, Zanotti-Gerosa A, Pettman A, Liu JK, Mills AJ, Xiao JL (2008) Chem Eur J 14:2209
Wu XF, Liu JK, Li XH, Zanotti-Gerosa A, Hancock F, Vinci D, Ruan JW, Xiao JL (2006) Angew Chem Int Ed 45:6718
Narayan S, Muldoon J, Finn MG, Fokin VV, Kolb HC, Sharpless KB (2005) Angew Chem Int Ed 44:3275
Baleizao C, Corma A, Garcia H, Leyva A (2004) J Org Chem 69:439
Corma A, Das D, Garcia H, Leyva A (2005) J Catal 229:322
Grushin VV, Alper H (1994) Chem Rev 94:1047
Hassan J, Hathroubi C, Gozzi C, Lemaire M (2001) Tetrahedron 57:7845
Liu RL, Shi YF, Wan Y, Meng Y, Zhang FQ, Gu D, Chen ZX, Tu B, Zhao DY (2006) J Am Chem Soc 128:11652
Wang SY, Moon SH, Vannice MA (1981) J Catal 71:167
Zhao DY, Huo QS, Feng JL, Stucky GD (1998) J Am Chem Soc 120:6024
Meng Y, Gu D, Zhang FQ, Shi YF, Cheng L, Feng D, Wu ZX, Chen ZX, Wan Y, Stein A, Zhao DY (2006) Chem Mater 18:4447
Thomas AC (1975) In: Photoelectron and auger spectroscopy, 1st edn. Plenum, New York
Wu YY, Livneh T, Zhang YX, Cheng GS, Wang JF, Tang J, Moskovits M, Stucky GD (2004) Nano Lett 4:2337
Wan Y, Wang HY, Zhao QF, Klingstedt M, Terasaki O, Zhao DY (2009) J Am Chem Soc 131:4541
Zhuang X, Wan Y, Feng CM, Shen Y, Zhao DY (2009) Chem Mater 21:706
Wan Y, Zhang DQ, Zhai YP, Feng CM, Chen J, Li HX (2007) Chem Asian J 2:875
Wan Y, Chen J, Zhang DQ, Li HX (2006) J Mol Catal A: Chem 258:89
Gomez-Quero S, Cardenas-Lizana F, Keane MA (2008) Ind Eng Chem Res 47:6841
Acknowledgements
This work was supported by NSF of China (20873086 and 20821140537), Shanghai Sci. & Tech. and Edu. Committee (07QH14011, 07SG49, S30406, 0852nm0090 and 08JC1417100), and the program for New Century Excellent Talents in Universities (NCET-07-0560).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Wan, Y. Synthesis of ordered mesoporous Pd/carbon catalyst with bimodal pores and its application in water-mediated Ullmann coupling reaction of chlorobenzene. J Mater Sci 44, 6553–6562 (2009). https://doi.org/10.1007/s10853-009-3612-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-009-3612-7