Abstract
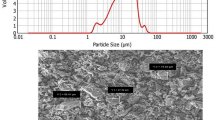
Open cell Ti6Al4V foams (60% porosity) were prepared at sintering temperatures between 1,200 and 1,350 °C using ammonium bicarbonate particles (315–500 μm) as space holder. The resulting cellular structure of the foams showed bimodal pore size distribution, comprising macropores (300–500 μm) and micropores (1–30 μm). Compression tests have shown that increasing sintering temperature increased the elastic modulus, yield and compressive strength, and failure strain of foams. The improvements in the mechanical properties of foams prepared using smaller size Ti64 powder with bimodal particle distribution were attributed to the increased number of sintering necks and contact areas between the particles. Finally, the strength of foams sintered at 1,350 °C was found to satisfy the strength requirement for cancellous bone replacement.















Similar content being viewed by others
References
Long M, Rack HJ (1998) Biomaterials 19:1621
Pilliar RM (1987) J Biomed Mater Res-Appl Biomater 21:1
Hulbert SF, Young FA, Mathews RS, Klawitter JJ, Talbert CD, Stelling FH (1970) J Biomed Mater Res 4:433
Bobyn JD, Miller JE (1994) Features of biologically fixed devices. American Academy of Orthopaedic Surgeons, Chicago
Oh IH, Nomura N, Hanada S (2002) Mater Trans 43:443
Wen CE, Yamada Y, Shimojima K, Chino Y, Asahina T, Mabuchi M (2002) J Mater Sci-Mater Med 13:397
Assad M, Likibi F, Jarzem P, Leroux MA, Coillard C, Rivard CH (2004) Materialwiss Werkstofftech 35:219
ASTM F 1580-95, Standard specification for titanium and Ti6Al4V alloy powders for coating surgical implants.
Martin B, Stiller C, Buchkremer HP, Stöver D, Baur H (2000) Adv Eng Mater 2:196
Rho JY, Kuhn-Spearing L, Zioupos P (1998) Med Eng Phys 20:92
Tasdemirci A, Hızal A, Altındis M, Hall IW, Guden M (2008) Mater Sci Eng A 474:335
Guden M, Celik E, Cetiner S, Aydin A (2004) In: Biomaterials: from molecules to engineered tissues, p 257
Tencer F, Johnson KD (1994) In: Biomechanics in orthopaedic trauma: bone fracture and fixation. Martin Dunitz Ltd, London, p 954
Burstein AH, Reilly DT, Martens M (1976) J Bone Joint Surg 58A:82
Chang YS, Oka M, Kobayashi M, Gu HO, Li ZL, Nakamura T, Ikada Y (1996) Biomaterials 17:1141
Acknowledgement
The authors would like to thank the Technology Development Foundation of Turkey (TTGV) for the grant #TTGV-102/T13.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dizlek, M.E., Guden, M., Turkan, U. et al. Processing and compression testing of Ti6Al4V foams for biomedical applications. J Mater Sci 44, 1512–1519 (2009). https://doi.org/10.1007/s10853-008-3038-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-008-3038-7