Abstract
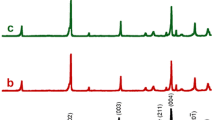
A series of Ca3−xGdxCo4O9+δ precursor powders were synthesized by the polyacrylamide gel method, and their ceramics were obtained by the Spark Plasma Sintering (SPS). There were lots of defects in the sheet-like grains from SEM and TEM observations. The electrical and the thermal transport properties were obviously affected by the material microstructure. The small polaron hopping conduction mechanism was determined above 600 K, and the hopping activation energy increased with the increase of doping contents. It was found that the Seebeck coefficient and the resistivity of doped samples were markly enhanced due to the impurity compensation effect, and their thermal conductivities were decreased due to the defects scattering. The maximum figure of merit of ZT = 0.24 at 973 K was obtained for Ca2.7Gd0.3Co4O9+δ.








Similar content being viewed by others
References
Park K, Kim KK, Seong JK (2007) Mater Lett 61:4759. doi:https://doi.org/10.1016/j.matlet.2007.03.021
Zhang LH, Tosh T, Norlyuki O et al (2007) Mater Trans 48:2088. doi:https://doi.org/10.2320/matertrans.E-MRA2007836
Park K, Ko KY, Seong JK et al (2007) J Eur Ceram Soc 27:3735. doi:https://doi.org/10.1016/j.jeurceramsoc.2007.02.030
Yasukawa M, Itoh S, Kono T (2005) J Alloy Compd 390:250. doi:https://doi.org/10.1016/j.jallcom.2004.07.061
Androulakis J, Hsu KF, Pcionek R (2006) Adv Mater 18:1170. doi:https://doi.org/10.1002/adma.200502770
Terasaki I, Sasago Y, Uchinokura K (1997) Phys Rev B 56:R12685. doi:https://doi.org/10.1103/PhysRevB.56.R12685
Masset AC, Michel C, Maignan A et al (2000) Phys Rev B 62:166. doi:https://doi.org/10.1103/PhysRevB.62.166
Koshibae W, Tsutsui K, Maekawa S (2000) Phys Rev B 62:6869. doi:https://doi.org/10.1103/PhysRevB.62.6869
Shikano M, Funahashi R (2003) Appl Phys Lett 82:1851. doi:https://doi.org/10.1063/1.1562337
Creon N, Perez O, Hadermann J (2006) Chem Mater 18:5355. doi:https://doi.org/10.1021/cm061163a
Prevel M, Perez O, Noudem JG (2007) Solid State Sci 9:231. doi:https://doi.org/10.1016/j.solidstatesciences.2007.01.003
Matsubara I, Funahashi R, Tomonari T (2001) J Appl Phys 90:462. doi:https://doi.org/10.1063/1.1378056
Asahi R, Sugiyama J, Tani T (2002) Phys Rev B 66:155103. doi:https://doi.org/10.1103/PhysRevB.66.155103
Wang DL, Chen LD, Wang Q et al (2004) J Alloy Compd 376:58. doi:https://doi.org/10.1016/j.jallcom.2003.12.018
Kobayashi T, Takizawa H, Endo T (1991) J Solid State Chem 92:116. doi:https://doi.org/10.1016/0022-4596(91)90248-G
Wang Y, Nyrissa S, Cava RJ et al (2003) Nature 423:425. doi:https://doi.org/10.1038/nature01639
Takeuchi T (2004) Phys Rev B 69:125410. doi:https://doi.org/10.1103/PhysRevB.69.125410
Acknowledgements
The authors would like to thank the financial supports from National Basic Research Program of China (973 program) under Grant No. 2007CB607502, the National Natural Science Foundation of China (NSFC) of No. 50801054 and 50772026, and Natural Science Key Fund of Heilongjiang Province in China (grant No. ZJG0605-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, H.Q., Zhao, X.B., Liu, F. et al. Effect of Gd-doping on thermoelectric properties of Ca3Co4O9+δ ceramics. J Mater Sci 43, 6933–6937 (2008). https://doi.org/10.1007/s10853-008-2990-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-008-2990-6