Abstract
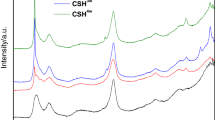
Hydration of tricalcium silicate in hydrothermal conditions in the presence of magnesium oxide has shown changes in the formation of CSH gel structure (Calcium silicate hydrates). The new CSH incorporates magnesium ions, brucite, but a weak presence of portlandite. The magnesium oxide would hinder the precipitation of portlandite. The characterization of CSH gel by 29Si MAS-NMR with various CaO/SiO2 ratios would point out that: (1) A dreierketten structure of the CSH for low CaO/SiO2 < 1, with some defects (Q3 defect and Q2v) in its structure is confirmed. Some magnesium ions are incorporated in the octahedral sites, in the interlayer space of the dreierketten pattern. (2) For the CSH gels with CaO/SiO2 ratios > 1, magnesium ions would be incorporated in the silicate chains of the CSH gel in a tetrahedral coordination. Although, the low MgO/CaO ratios of CSH gels indicate that the magnesium incorporation in CSH chain is low.







Similar content being viewed by others
References
Taylor HFW (1990) Cement chemistry. Academic Press, London
Masse S (1993) PhD: Synthèse Hydrothermal d’Hydrates de Silicate Tricalcique. Analyse Structurale en Phase Solide. Etude Comparative avec les Ciments Utilisés pour Chemiser les Puits de Pétrole, Université Pierre et Marie Curie Paris
Flint EP, Wells LS (1934) J Res Natl Bur Stand 12:751
Brunauer S, Kantro D, Copeland L (1958) J Am Chem Soc 80:761. doi:https://doi.org/10.1021/ja01537a001
Greenberg SA, Chang TN (1965) J Phys Chem 69:182. doi:https://doi.org/10.1021/j100885a027
Greenberg SA, Chang TN, Anderson E (1965) J Phys Chem 64:1151. doi:https://doi.org/10.1021/j100838a012
Roller PS, Ewin JG (1940) J Am Chem Soc 62:461. doi:https://doi.org/10.1021/ja01860a001
Taylor HFW (1950) J Chem Soc 726:3682. doi:https://doi.org/10.1039/JR9500003682
Nonat A (2004) Cem Concr Res 34:1521. doi:https://doi.org/10.1016/j.cemconres.2004.04.035
Richardson IG (2004) Cem Concr Res 34:1733. doi:https://doi.org/10.1016/j.cemconres.2004.05.034
Cong X, Kirkpatrick R (1996) J Adv Cem Base Mater 3:144. doi:https://doi.org/10.1016/S1065-7355(96)90046-2
Klur I (1996) PhD: Etude par RMN de la Structure des Silicates de Calcium Hydratés, Université Pierre et Marie Curie Paris
Klur I, Pollet B, Virlet J, Nonat A (1998). In: Colombet P (ed) Nuclear magnetic resonance spectroscopy of cement-based materials. Springer, Berlin
Grutzeck M, Benesi A, Fanning B (1989) J Am Ceram Soc 72:665. doi:https://doi.org/10.1111/j.1151-2916.1989.tb06192.x
Rodger SA, Groves GW, Clayden NJ, Dobson CM (1988) J Am Ceram Soc 71:91. doi:https://doi.org/10.1111/j.1151-2916.1988.tb05823.x
Fernandez L, Alonso C, Hidalgo A, Andrade C (2005) Adv Cem Res 17:9. doi:https://doi.org/10.1680/adcr.17.1.9.58392
Pytel Z, Malolepszy J (1997). In: Justnes H (ed) Proc Int Congr Chem Cem 10th, Amarkai AB, Goeteborg
Shrivastava OP, Komarneni S, Breval E (1991) Cem Concr Res 21:83. doi:https://doi.org/10.1016/0008-8846(91)90034-F
Xu G, Lai Z, Qian G, Yang S, Zhou Q (2000) Guisuanyan Xuebao 28:100
Massiot D, Thiele H, Germanus A (1994) Bruker Rep 140:43
Engelhardt G, Michel D (1987) High-resolution solid-state NMR of silicates and zeolites. Wiley & Sons, Chichester
Mägi M, Lippmaa E, Samoson A, Engelhardt G, Grimmer AR (1984) J Phys Chem 88:1518. doi:https://doi.org/10.1021/j150652a015
Brough AR, Dobson CM, Richardson IG, Groves GW (1994) J Am Ceram Soc 77:593. doi:https://doi.org/10.1111/j.1151-2916.1994.tb07034.x
Cong X, Kirkpatrick RJ (1993) Cem Concr Res 23:1065. doi:https://doi.org/10.1016/0008-8846(93)90166-7
Dobson CM, Goberdhan DGC, Ramsay JDF, Rodger SA (1988) J Mater Sci 23:4108. doi:https://doi.org/10.1007/BF01106844
Noma H, Adachi Y, Yamada H, Nishino T, Matsuda Y, Yokoyama T (1998). In: Colombet P (ed) Nuclear magnetic resonance spectroscopy of cement-based materials. Springer, Berlin
Ramanchandran VS, Feldman RF, Beaudoin JJ (1981) Concrete science. Heyden and Son Ltd., Philadelphia, PA
Taylor HFW (1986) J Am Ceram Soc 69:464. doi:https://doi.org/10.1111/j.1151-2916.1986.tb07446.x
Stein HN, Stevels JM (1964) J Appl Chem 14:338
Grutzeck MW, Kwan S, Thompson JL, Benesi A (1999) J Mater Sci Lett 18:217. doi:https://doi.org/10.1023/A:1006624215448
Barnes JR, Clague ADH, Clayden NJ, Dobson CM, Hayes CJ, Groves GW et al (1985) J Mater Sci Lett 4:1293. doi:https://doi.org/10.1007/BF00723485
Brunet F, Bertani P, Charpentier T, Virlet J, Nonat A (2004) J Phys Chem B 108:15494. doi:https://doi.org/10.1021/jp031174g
Klur I, Jacquinot JF, Brunet F, Charpentier T, Virlet J, Schneider C et al (2000) J Phys Chem B 104:10162. doi:https://doi.org/10.1021/jp001342u
Faucon P, Delaye JM, Virlet J (1996) J Solid State Chem 127:92. doi:https://doi.org/10.1006/jssc.1996.0361
Faucon P, Jacquinot JF, Delaye JM, Virlet J (1997) Philos Mag B 75:769. doi:https://doi.org/10.1080/13642819708202353
Skibsted J, Jakobsen HJ, Hall C (1995) J Chem Soc-Faraday Trans 91:4423. doi:https://doi.org/10.1039/ft9959104423
Edwards CL, Alemany LB, Barron AR (2007) Ind Eng Chem Res 46:5122. doi:https://doi.org/10.1021/ie070220m
Stephan D, Wistuba S (2006) J Eur Ceram Soc 26:141. doi:https://doi.org/10.1016/j.jeurceramsoc.2004.10.031
Peterson VK, Hunter BA, Ray A (2004) J Am Ceram Soc 87:1625
De La Torre AG, Bruque S, Campo J, Aranda MAG (2002) Cem Concr Res 32:1347. doi:https://doi.org/10.1016/S0008-8846(02)00796-2
De La Torre AG, Bruque S, Aranda MAG (2001) J Appl Crystallogr 34:196. doi:https://doi.org/10.1107/S0021889801002485
Mumme WG (1995) Neues Jahrb Mineral-Montash Hefte 4:145
Clayden NJ, Dobson CM, Groves GW, Rodger SA (1986). In: Secr (ed) Congr Int Quim Cimento 8th, Geral 8o CIQC, Rio de Janeiro
Janes N, Oldfield E (1985) J Am Chem Soc 107:6769. doi:https://doi.org/10.1021/ja00310a004
Dupree R, Smith ME (1988) J Chem Soc Chem Commun 22:1483. doi:https://doi.org/10.1039/C39880001483
MacKenzie KJD, Meinhold RH (1994) J Mater Chem 4:1595. doi:https://doi.org/10.1039/jm9940401595
Fiske PS, Stebbins JF (1994) Am Mineral 79:848
Yang H, Hazen RM, Downs RT, Finger LW (1997) Phys Chem Miner 24:510. doi:https://doi.org/10.1007/s002690050066
Acknowledgement
The authors thank the “Ministerio de Educación y Ciencia” and the C.I.C.Y.T of Spain for the funds provided, as well as to the DG-XII of the E.U: UNICORN Project (BRPR-CT97-0511). We also thank to Dr. C. Vernet (Lafarge Society) for the synthesis of C3S and to the Department of NMR. Spectroscopy (C.A.I) from the “Universidad Complutense de Madrid” for the testing facilities.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Fernandez, L., Alonso, C., Andrade, C. et al. The interaction of magnesium in hydration of C3S and CSH formation using 29Si MAS-NMR. J Mater Sci 43, 5772–5783 (2008). https://doi.org/10.1007/s10853-008-2889-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-008-2889-2