Abstract
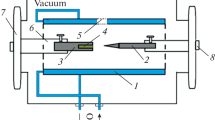
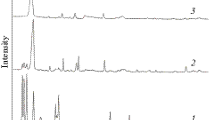
Nanosized tungsten carbide powder was prepared by a thermal plasma process using tungsten hexachloride (WCl6) as the precursor. The reduction and carburization of the vaporized precursor by methane–hydrogen mixtures produced nanosized WC1−x powder, which sometimes contained WC and/or W2C phase. The effects of the molar ratio of reactant gases, plasma torch power, the flow rate of plasma gas, and the addition of secondary plasma gas (H2) on the product composition and grain size were investigated. The tungsten carbide powder produced by the plasma process showed particle sizes less than 20 nm. The produced powder was heated in hydrogen to fully carburize the WC1−x, and W2C phases to the WC phase as well as to remove excess carbon in the product. Finally, WC powder with particle size less than 100 nm was obtained.












Similar content being viewed by others
References
Upadhaya GS (2002) Cemented tungsten carbide. Noyes Publications, New York
Petersson A, Ågren J (2004) Acta Mater 52:1847. doi:https://doi.org/10.1016/j.actamat.2003.12.024
Fang Z, Maheshwari P, Wang X et al (2005) Inter J Refract Metab Hard Mater 23:249. doi:https://doi.org/10.1016/j.ijrmhm.2005.04.014
Lee G-H, Kang S (2006) J Alloy Comp 419:281. doi:https://doi.org/10.1016/j.jallcom.2005.09.060
Wahlberg S, Grenthe I, Muhammed M (1997) Nanostruct Mater 9:105. doi:https://doi.org/10.1016/S0965-9773(97)00029-9
Zhu YT, Manthiram A (1996) Compos Part B Eng 27:407
Fu L, Cao LH, Fan YS (2001) Scr Mater 44:1061. doi:https://doi.org/10.1016/S1359-6462(01)00668-6
Nersisyan HH, Won HI, Won CW et al (2005) Mater Chem Phys 94:153. doi:https://doi.org/10.1016/j.matchemphys.2005.04.024
Wu XY, Zhang W, Wang W et al (2004) J Mater Res 19:2240. doi:https://doi.org/10.1557/JMR.2004.0324
Zawrah MF (2007) Ceram Int 33:155. doi:https://doi.org/10.1016/j.ceramint.2005.09.010
Shi XL, Shao GQ, Duan XL et al (2006) Mater Charact 57:358. doi:https://doi.org/10.1016/j.matchar.2006.02.013
McCandlish LE, Kear BH, Kim BK (1992) Nanostruct Mater 1:119. doi:https://doi.org/10.1016/0965-9773(92)90063-4
Ban Z-G, Shaw LL (2002) J Mater Sci 37:3397. doi:https://doi.org/10.1023/A:1016553426227
Hasanpour A, Mozaffari M, Amighian J (2007) Physica B (Amsterdam) 387:298. doi:https://doi.org/10.1016/j.physb.2006.04.039
Liu S, Huang Z-L, Liu G et al (2006) Inter J Refract Metab Hard Mater 24:461. doi:https://doi.org/10.1016/j.ijrmhm.2005.10.001
Mi S, Courtney TH (1997) Scr Mater 38:171. doi:https://doi.org/10.1016/S1359-6462(97)00410-7
Chang W, Skandan G, Hahn H et al (1994) Nanostruct Mater 4:345. doi:https://doi.org/10.1016/0965-9773(94)90144-9
Tong L, Reddy RG (2005) Scr Mater 52:1253. doi:https://doi.org/10.1016/j.scriptamat.2005.02.033
Moriysohi Y, Futaki M, Komatsu S et al (1997) J Mater Sci Lett 16:347. doi:https://doi.org/10.1023/A:1018586009506
Fukumasa O, Fujiwara T (2003) Thin Solid Films 435:33. doi:https://doi.org/10.1016/S0040-6090(03)00371-7
Swihart MT (2003) Curr Opin Colloid In 8:127. doi:https://doi.org/10.1016/S1359-0294(03)00007-4
Gao Y, Guo X-P, Wei R (2006) Surf Coat Tech 201:2829. doi:https://doi.org/10.1016/j.surfcoat.2006.05.035
Tong L, Reddy RG (2006) Mater Res Bull 41:2303. doi:https://doi.org/10.1016/j.materresbull.2006.04.021
Mohai I, Gál L, Szépvölgyi J et al (2007) J Eur Ceram Soc 27:941. doi:https://doi.org/10.1016/j.jeurceramsoc.2006.04.128
Hojo J, Oku T, Kato A (1978) J Less Common Met 59:85. doi:https://doi.org/10.1016/0022-5088(78)90114-5
Fitzsimmons M, Sarin VK (1995) Surf Coat Tech 76:250
Kim JC, Kim BK (2004) Scr Mater 50:969. doi:https://doi.org/10.1016/j.scriptamat.2004.01.015
Tang X, Haubner R, Lux B et al (1995) J Phys II 5:1013. doi:https://doi.org/10.1051/jp3:1995174
Won C-W, Chun B-S, Sohn HY (1993) J Mater Res 8:2702. doi:https://doi.org/10.1557/JMR.1993.2702
Leclercq G, Kamal M, Giraudon JM et al (1996) J Catal 158:142. doi:https://doi.org/10.1006/jcat.1996.0015
Kelly CM, Garg D, Dyer PN (1992) Thin Solid Films 219:103. doi:https://doi.org/10.1016/0040-6090(92)90729-U
Medeiros FFP, Oliveira SAD, Souza CPD et al (2001) Mater Sci Eng A 315:58. doi:https://doi.org/10.1016/S0921-5093(01)01214-X
Gao L, Kear BH (1995) Nanostruct Mater 5:555. doi:https://doi.org/10.1016/0965-9773(95)00265-G
Cullity BD (1978) Elements of X-ray diffraction, 2nd edn. Addison-Wesley Pub. Co, London
Sara RW (1965) J Am Ceram Soc 48:253
Choi SI, Nam JS, Lee CM et al (2006) Curr Appl Phys 6:224. doi:https://doi.org/10.1016/j.cap.2005.07.045
Acknowledgements
This material is based upon the work supported by the US Department of Energy under Award No. DE-FC36-04GO14041 with cost sharing by Kennametal and Smith International and technical collaboration with Idaho National Laboratory. The authors wish to thank Prof. Patrick R. Taylor of Colorado School of Mines for his help with the selection, design, and initial operation of the plasma reactor system. Thanks also go to Mr. Robert W. Byrnes of the University of Utah for his competent work with the design and repair of the experimental facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ryu, T., Sohn, H.Y., Hwang, K.S. et al. Tungsten carbide nanopowder by plasma-assisted chemical vapor synthesis from WCl6–CH4–H2 mixtures. J Mater Sci 43, 5185–5192 (2008). https://doi.org/10.1007/s10853-008-2741-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-008-2741-8