Abstract
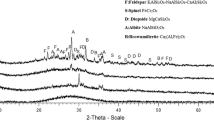
Crystallization behavior of a waste-based glass-ceramic was studied by means of X-ray diffraction analysis, and the surface morphological observations and chemical compositions were evaluated by field emission-scanning electron microscopy and energy dispersive X-ray spectrometry. Applying the mechanical milling method, the glass-ceramic was prepared by using fly ash from a thermal power plant mixed with waste glass cullet. Powder mixtures consisting of waste glass powder (70 wt%) and fly ash (30 wt%) were used to make glass-ceramic. Various heat treatment temperatures [900, 925, 950, 975, 1000 and 1025°C] were used to obtain a glass-ceramic of the optimum crystal phase, mechanical properties and chemical durability. The X-ray diffraction analysis showed that the crystalline phases in the glass-ceramic were diopside [Ca(Mg, Al)(Si, Al)2O6], augite [Ca(Mg, Fe)Si2O6] and wollastonite [CaSiO3]. The crystallization of an acicular phase in the matrix was achieved in the heat treatment temperature range of 1000–1025°C, and the acicular type main crystal phase in the glass-ceramic was wollastonite [CaSiO3]. The heat treatment temperature range [1000–1025°C] also showed much better mechanical properties.
Similar content being viewed by others
References
D. HERMAN, Ceram. Inter. 24 (1998) 515.
Korea Hydro & Nuclear Power Co., Ltd. South Korea (2002).
U. PIETRO and D. CALABRESE, Thermochim. Acta. 321 (1998) 143–150.
R. DERIE, Waste Manage. 16 (1996) 711.
M. EROL, S. KUCUKBAYRAK, A. ERSOY-MERICBOYU and M. L. OVECOGLU, J. Eur. Ceram. Soc. 21 (2001) 2835.
R. CIOFFI, P. PERNICE, A. ARONNE and G. QUATTRONI, J. Mater. Sci. 28 (1993) 6591.
E. J. DE GUIVE and S. H. RISBUD, J. Mater. Sci. 19 (1994) 1760.
A. A. FRANCIS, R. D. RAWLINGS, R. SWEENEY and A. R. BOCCACCINI, Glass Technol. 43 (2002) 58.
An Environmental White Paper, The Ministry of Environment, South Korea (2000).
A. R. BOCCACCINI, M. BUèCKER, J. BOSSERT and K. MARSZALEK, Waste Manage. 17 (1997) 39.
L. BARBIERI, A. CORRADI and I. LANCELLOTTI, J. Eur. Ceram. Soc. 20 (2000) 1637.
Y. H. YUN, C. H. YOON, J. S. OH, S. B. KIM, B. A. KANG, and K. S. HWANG, J. Mater. Sci. 37 (2002) 3211.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoon, SD., Yun, YH. Waste glass and fly ash derived glass-ceramic. J Mater Sci 41, 4315–4319 (2006). https://doi.org/10.1007/s10853-006-7019-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-7019-4